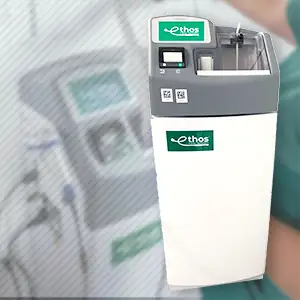
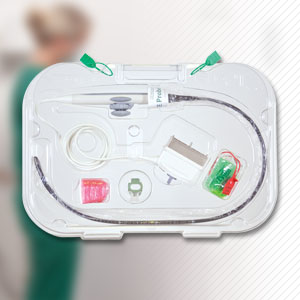
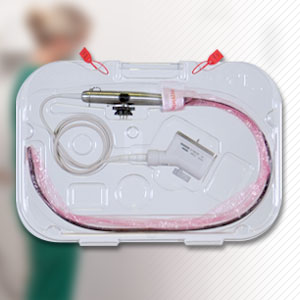
Products
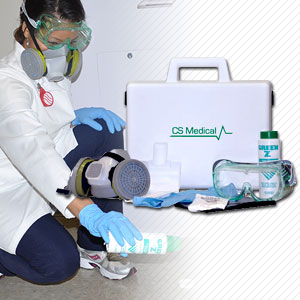
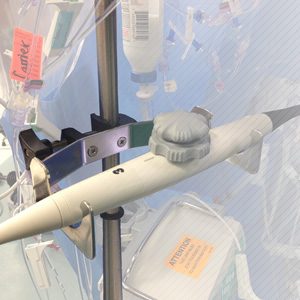
Device Disinfection
Device Care & Storage
21
Years of Experience
3 or 5
Minute Disinfection Time
8
Over Eight Million
High-Level Disinfections
High-Level Disinfections
0
FDA Medical Device Reports
Schedule a time to speak with one of our sales professionals
Request a Product Price Quote today