Proper Cleaning: How It Affects the Outcome of High Level Disinfection
Introduction
Evidence has been presented that semi-critical devices, devices that come in contact with mucous membranes or non-intact skin, such as endoscopes and endocavity probes, cause more healthcare associated infections than non-critical or critical medical devices. The number of infections from semi-critical devices is partially accounted for by the narrow margin of safety associated with reprocessing these devices by cleaning and high level disinfection (HLD)1. Therefore, any deviation from recommended protocols can hinder HLD and leave a device contaminated with microorganisms. One critical step to achieving adequate HLD is proper cleaning of devices prior to disinfection. Cleaning is the process by which soil and contaminants are physically removed from an instrument. Cleaning can be done using manual or newer automated methods. However, automated cleaning is often the better choice because it achieves the same levels of cleanliness, but reduces human error and increases compliance with reprocessing protocols to decrease the risk of contaminated devices being used for patient procedures.
Soil Levels on Endoscopes and Endocavity Probes
After use, endoscopes and endocavity probes are heavily contaminated with microorganisms and other organic material. Microbial load, protein, hemoglobin, and ATP are the most commonly used markers for soil levels on endoscopes because they can be easily measured and represent common materials a scope may come in contact with in the body. Endoscopes can be contaminated with up to 1 billion microorganisms2-s and over 200mg of protein2-5• However, it is important to note that microbial load and pre-cleaning soil levels vary greatly between endoscope types and individual devices. Differences in soil levels between endoscope types can probably be attributed to differences in how these endoscopes are used and the number of microorganisms in the body cavities in which they are used. For example, bronchoscopes tend to be contaminated with fewer microorganisms and less protein than colonoscopes. This is to be expected because the lower gastrointestinal tract, where colonoscopes are used, contains many more microorganisms than the throat or lungs. Further, a wide range in the number of microorganisms or soil recovered from individual endoscopes has been reported. Much of this variability likely represents differences in sampling and recovery methods, as well as other clinical or patient factors.
Despite high levels of microorganism and soil contamination on endoscopes after patient procedures, optimum cleaning alone can reduce microbial contamination by 4-6 logs2•6-8• In most instances, optimum cleaning is sufficient to reduce hemoglobin and protein by greater than 95% (-1.3 log reduction)3. Using observations of the pre- and post-cleaning soil levels on endoscopes, the field has defined what constitutes a "clean" endoscope using microbial load, protein, hemoglobin, and ATP levels as metrics. Currently, the most widely accepted benchmarks are: a microbial load of 100 CFU, 6.4µg/cm2 protein, 2.2µg/cm hemoglobin, and 200 RLU of ATP2-4_ It is important to note these benchmarks were defined using older cleaning protocols. As new and better cleaning methods have become available, these benchmarks have become easy to attain, provided cleaning is done properly. Some suggest new recommended cleaning procedures and propose lowering the benchmarks for soil levels on clean endoscopes to a microbial load of less than 100cfu/cm2, less than 2µg/cm2 protein, and less than 200 RLU of ATP3. It will be up to the field to decide whether or not to accept these new proposed cleanliness benchmarks, keep the current benchmarks, or define new benchmarks entirely.
Manual vs. Automatic Cleaning
Proper cleaning of endoscopes and endocavity probes can significantly reduce soil levels prior to HLD. However, the process of cleaning these medical devices following their use can be tedious, confusing, and lacks standardization. Standardization via automation of HLD is used in many clinical settings, however, automated cleaning of endoscopes and endocavity probes has only become recently available. In a controlled laboratory environment, automated cleaning performs similarly to optimum manual cleaning. Both cleaning methods met the standard requirements for clean (<6.4µg / cm2 protein,>99.9% reduction hemoglobin, and 2-4 log reduction in bioburden), suggesting that under optimum conditions there is little difference between manual and automated cleaning9•

However, clinical settings often do not provide optimum conditions. Thus, though manual and automated cleaning can achieve similar levels of clean in a laboratory environment, automatic cleaning is better suited to the suboptimal clinical environments in which endoscopes and endocavity probes are cleaned and reprocessed10• Manual cleaning of endoscopes and endocavity probes is a long and tedious process. Each step must be carefully completed to ensure proper cleaning and disinfection without damaging the device. If you also consider the need to reprocess probes efficiently and in a timely manner, the reprocessing procedure quickly becomes rife with human error. One study found that only 1.4% of endoscopes reprocessed using a manual cleaning method and automated disinfection cycle were reprocessed correctly at every step11• Many of the errors made occurred during the manual cleaning stage and drying after reprocessing. Further support for suboptimal manual cleaning conditions in the clinic comes from observations of clinic cleaning times. In the study comparing manual with automated cleaning methods discussed above, scientists spent an average of 14-25 min manually cleaning probes, whereas the average time spent cleaning endoscopes in a clinic is about 4-7 min9• This supports the notion that manual cleaning procedures completed in clinics may not be as stringent as those in a laboratory environment.
There are many reasons why manual cleaning procedures remain suboptimal in clinical settings. Manual cleaning of endoscopes and endocavity probes can be an arduous process. Clinic staff report pain, decreased flexibility, numbness/tingling, and physical discomfort associated with the tedium of manual reprocessing of endocavity probes and endoscopes11• Given the physical toll of manual cleaning, it is easy to see how steps may be shortened or skipped entirely, particularly if staff are required to perform multiple reprocessing procedures sequentially. Reprocessing procedures can also vary between and within healthcare facilities. 92% of infection prevention professionals report wanting to standardize processes for reprocessing ultrasound probes in their facilities 2 procedures not only between facilities, but also within a given facility. The variability in cleaning between probe types also plays a factor and adds to the challenge. Some probes, with the aid of adaptors, can be completely submerged, while others, like TEE probes, cannot be completely submerged. Making sure you do not get specific parts of the probe wet, thus ruining the probe, adds to the stress of manual cleaning. This increases the complexity of knowing all the probes and cleaning methods for each. Whether protocols differ by department, staff, probe manufacturer, or the infection status of the patient, the end result can be confusion about HLD processes. Protocol changes for devices used in infected patients also reinforces an incorrect notion that instruments used on patients without infections are less contaminated or pose less threat than instruments used on patients with known infections. Lastly, endoscope or probe reprocessing must occur in a fairly efficient manner to ensure an instrument is available to use at all times. Depending upon the number of instruments, trained staff, and procedures performed at a particular location, there may only be a short period of time available for probes to be reprocessed13• Time constraints could increase the chance that steps are skipped in the reprocessing procedure and instruments are not properly cleaned before disinfection.
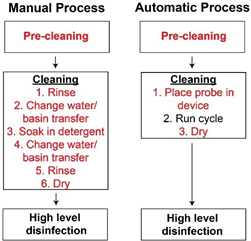
Multiple solutions have been proposed to combat the issues associated with manual cleaning. Enzymatic cleaning wipes or sponges for endocavity ultrasound probes are commercially available solutions to simplify the cleaning process. Prepackaged wipes or sponges can reduce the number of steps involved in cleaning and potentially reduce cleaning times by eliminating the need to soak probes in detergent. However, they require that the user evenly wipe the surface of the device, leaving the potential risk that small areas of the device could be missed. Therefore, complete automated cleaning of endoscopes and endocavity probes is likely the best solution to reduce failures associated with reprocessing. Automated cleaners and disinfectors, such as the TEEClean®Automated TEE Probe Cleaner Disinfector, require users to pre-clean devices before finishing the process by placing them in the automated cleaning device. This minimizes the amount of physical washing and basin transfers that must be completed by the user; thus reducing the number of steps subject to human error, while reducing the physical demands on staff. Indeed, compliance with automated cleaning and disinfection procedures was observed to be much higher (75.4%) than that of manual cleaning (1.4%)11• Further, automated cleaning procedures are designed to remain consistent between runs, eliminating possible changes in the cleaning protocol resulting from error or differences in staff and training. Each probe is cleaned and disinfected under the assumption it is contaminated with potentially harmful microbes to adhere to standard precautions in healthcare14• Automated cleaning takes 7-10 min and requires little hands-on time by a technician. Less hands-on time helps alleviate issues with short staffing or heavy workload in healthcare settings where few personnel are trained on reprocessing procedures. Therefore, automated cleaning devices can meet the time constraints and reprocessing efficiency required in a clinical setting without compromising patient safety.
Consequences of Improper Cleaning on HLD and Patient Safety
Improper cleaning of reusable medical devices leads to inadequate disinfection. When devices are not cleaned correctly, remaining soil on the device can decrease the effectiveness of the subsequent disinfection cycle by physically preventing the disinfectant from making contact with the surface of the device8•15•16• Depending upon the disinfectant and type of soil, the remaining soil on improperly cleaned devices can also bind or inactivate the disinfectant, making it less effective in killing contaminating microorganisms8•15•16• In a laboratory study, angioscopes that were not cleaned prior to disinfection still contained infectious duck hepatitis B virus (DHBV)17. In contrast, researchers found that even when the disinfectant contact time was reduced, infectious DHBV could not be recovered from properly cleaned devices17• In the clinic, cleaning and subsequent disinfection reduces the microbial load on colonoscopes from about 10 logs to 010• However, in the same study, failure to clean the colonoscopes prior to disinfection only reduced the microbial load to 3.8 log cfu/ml (a 5 log reduction)10• Together, these studies highlight the importance of proper cleaning prior to disinfection to ensure disinfectants work effectively.

Effective disinfection is critical to maintaining patient safety and avoiding infection from endoscopes and endocavity probes. In 2016, Joint Commission estimated that 74% of immediate threat to life declarations were related to improperly sterilized or high-level disinfected equipmentl 8. Outbreaks resulting from improperly disinfected endoscopes and endocavity probes have been reported worldwide19·23• In the US, an outbreak of Escherichia coli in 2006 was caused by contaminated TEE probes19• Subsequent investigation determined the probes were not disinfected or stored properly. Additionally, one probe was found to have a physical defect allowing for bacteria to accumulate and avoid disinfection. Use of contaminated TEE probes in two hospitals in Japan, led to outbreaks of multidrug-resistant Pseudomonas aeruginosa and Enterobacter cloacae, which resulted in one patient death20• 22• The P. aeruginosa outbreak was traced back to a physical defect in the probe22 However, it is suspected the outbreak of E. cloacae was likely caused by deviations in the cleaning and disinfection protocols because enforcement of disinfection procedures and implementation of single-use sheath covers reduced the number of E. cloacae infections in the hospital to baseline levels20•
The E.coli outbreak in the US and the outbreak of multidrug-resistant P. aeruginosa in Japan highlight the importance of manual inspection of the probe, as well as conducting electrical leakage tests between each use. Leakage tests not only alert the user to issues with the electrical workings of the device, but can also help identify breaches in the outer insulation barrier of the probe. Cracks or deformities in the outer barrier of the probe or scope can become contaminated with bacteria. Once bacteria are trapped within these areas, they tend to thrive for two reasons. First, detergents and disinfectants may not reach deep within cracks/deformities, allowing trapped bacteria to avoid killing. Second, moisture tends to be trapped within cracks or deformities, which further facilitates microbial growth. The end result is that even cleaned and disinfected probes remain contaminated with bacteria and put patients at risk for infection.
Fortunately, the E.coli and P. aeruginosa occurrences were localized to single facilities for limited times. In the case of widespread contamination of healthcare facilities and/or infection of patients, cleaning failure could have provided the path for uncontrolled pathogen transmission. This type of situation, where there is a prevalent pathogen plus a disinfection failure, was the underlying mechanism for the spread of HIV to hemophiliacs from 1980 to 1990. In that case, the pathogen was the HIV virus and the disinfection failure was a process shortcoming in hemostatic replacement factor manufacturing (e.g., see White, 201024). A current challenge regards whether or not widespread COVID-19 infection of critically ill patients undergoing TEE and various endoscopic procedures present a similar potential for widespread disease transmission.
Conclusion
Without proper cleaning and disinfection, a contaminated endoscope or endocavity probe serves as a vehicle for infection transmission between patients. However, meeting the logistical and physical demands of the manual cleaning process in clinics has proven difficult and has led to multiple outbreaks. Thus, there is a critical need to address issues surrounding endoscope and endocavity probe reprocessing. Automated cleaning procedures seem to be the solution. For reprocessing of TEE probes, the TEEClean®Automated TEE Probe Cleaner Disinfector is the first automated TEE ultrasound probe reprocessor cleared by the FDA that offers automated cleaning and disinfection in the same device. TEEClean®eliminates the physical demands of manual cleaning and maintains consistency in reprocessing procedures to ensure devices are reprocessed correctly each time. The limited hands-on time means a reproducibly clean probe can be achieved efficiently to meet the needs of a busy hospital. Thus, TEEClean offers a solution to reduce the risk of healthcare acquired infections from contaminated TEE probes in a reproducible and efficient manner.
References:
1. Rutala, W. A. & Weber, D.J. Disinfection and sterilization: An overview. AmJ Infect Control
41, S2-S5, doi:10.1016/j.
ajic.2012.11.005 (2013).
2. Alfa, M.J., Degagne, P. & Olson, N. Worst-case soiling levels for patient-used flexible
endoscopes before and after
cleaning. Am J Infect Control 27, 392-401, doi:https://doi.org/10.10l 6/S0196-6553(99)70004-0
(1999).
3. Alfa, M.J., Olson, N. & Murray, B.-L. Comparison of clinically relevant benchmarks and
channel sampling methods used to assess manual cleaning compliance for flexible gastrointestinal
endoscopes. Am J Infect Control 42, el-e5, doi:10.1016/j.
ajic.2013.08.007 (2014).
4. Alfa, M.J., Sepehri, S., Olson, N. & Wald, A. Establishing a clinically relevant bioburden
benchmark: a quality indicator for
adequate reprocessing and storage of flexible gastrointestinal endoscopes. AmJ Infect Control
40, 233-236, doi:10.1016/j. ajic.2011.02.023 (2012).
5. Alfa, M.J. & Olson, N. Physical and composition characteristics of clinical secretions
compared with test soils used for
validation of flexible endoscope cleaning. J Hosp Infect 93, 83-88,
doi:https://doi.org/10.1016/j.jhin.2016.01.023 (2016).
6. Chu, N. S., McAlister, D. & Antonoplos, P.A. Natural bioburden levels detected on flexible
gastrointestinal endoscopes
after clinical use and manual cleaning. Gastrointestinal Endoscopy 48, 137-142,
doi:10.1016/S0016-5107(98)70154-3 (1998).
7. Hanson, P. J. et al. Recovery of the human immunodeficiency virus from fibreoptic
bronchoscopes. Thorax 46, 410-412,
doi:l 0.1136/thx.46.6.410 (1991).
8. Rutala, W. A., Weber, D.J. & Committee, H. I. C. P.A. (ed Centers for Disease Control and
Prevention) 14-21 (2008).
9. Alfa, M.J., Olson, N. & DeGagne, P. Automated washing with the Reliance Endoscope Processing
System and its
equivalence to optimal manual cleaning. Amj Infect Control 34, 561-570,
doi:10.1016/j.ajic.2006.01.010 (2006).
10. Grenkova, T., Selkova, E., Aleshkin, V. & Melkumyan, A. Ensuring infectious safety of
colonoscopy. BMC Proceedings 5,
P309-P309, doi:10.1186/1753-6561-5-S6-P309 (2011).
11. Ofstead, C. L., Wetzler, H. P., Snyder, A. K. & Horton, R. A. Endoscope Reprocessing
Methods: A Prospective Study on
the Impact of Human Factors and Automation. Gastroenterol Nurs 33 (2010).
12. Carrico, R. M., Furmanek, S. & English, C. Ultrasound probe use and reprocessing: Results
from a national survey among
U.S. infection preventionists. Amj Infect Control 46, 913-920, doi:10.1016/j.ajic.2018.03.025
(2018).
13. SGNA practice committee. Standards of infection prevention in reprocessing flexible
gastrointestinal endoscopes. 1-34
(Society of Gastroenterology Nurses and Associates, Inc., Chicago, Illinois, 2018).
14. Division of Healthcare Quality Promotion. Guide to infection prevention for outpatient
settings: Minimum expectations
for safe care. 1-44 (Centers for Disease Control and Prevention, 2016).
15. Muscarella, L. F. Sterilizing dental equipment. Nat Med 1, 1223-1224,
doi:10.1038/nm1295-1223b (1995).
16. Parker, H. H. &Johnson, R. B. Effectiveness of ethylene oxide for sterilization of dental
handpieces.J Dent 23, 113-115,
doi:https://doi.org/10.1016/0300-5712(95)98977-B (1995).
17. Chaufour, X. et al. Evaluation of disinfection and sterilization of reusable angioscopes
with the duck hepatitis B model.J
Vase Surg 30, 277-282, doi:10.1016/S0741-5214(99)70138-2 (1999).
18.Joint Commission. Quick Safety, Improperly sterilized or HLD equipment. (Division of Health
Care Improvement) 1-3
(2017).
19. Bancroft, E. A., English, L., Terashita, D. & Yasuda, L. Outbreak of Escherichia coli
infections associated with a contaminated transesophageal echocardiography probe. Infect Control
Hosp Epidemiol 34, 1121-1123, doi:10.1086/673160
(2013).
20. Kanemitsu, K. et al. An increased incidence of Enterobacter cloacae in a cardiovascular
ward.J Hosp Infect 66, 130-134,
doi:https://doi.org/10.1016/j.jhin.2007.03.019 (2007).
21. Medicines and Healthcare Products Regulatory Agency. Medical device alert. MDA/2012/037. 1-4
(Crown Copyright,
United Kingdom, 2012).
22. Seki, M., Machida, H., Yamagishi, Y., Yoshida, H. & Tomono, K. Nosocomial outbreak of
multidrug-resistant Pseudomonas aeruginosa caused by damaged transesophageal echocardiogram
probe used in cardiovascular surgical
operations.J Infect Chemother 19, 677-681, doi:10.1007/s10156-012-0542-0 (2013).
23. Srinivasan, A. et al. An outbreak of Pseudomonas aeruginosa infections associated with
flexible bronchoscopes. N Englj
Med 348, 221-227, doi:10.1056/NEJMoa021808 (2003).
24. White, G. (2010). Hemophilia: An amazing 35 year journey from the depths of HIV to the
threshold of cure. Trans. Am.
Clin. And Climate. Assoc., 121, 61-75.
