After an ultrasound procedure, the transducer must be cleaned and disinfected prior to use on the next patient. The Spaulding Classification aides healthcare workers in determining the level of cleaning and disinfection the ultrasound probe must undergo before being returned for patient screening. Rinsing and Storage after cleaning and disinfection play an important role in the process of keeping patients safe and reducing the potential for HAIs.
Let’s start with what comes first after using an endocavity ultrasound probe on a patient.
Cleaning
Cleaning is a mandatory first step in the high-level disinfection process of endocavity ultrasound transducers. Once the procedure cover is removed, the next step is to clean the transducer. The terms “pre-cleaning” and “cleaning” are often used interchangeably in many healthcare settings. When considering what to do first, it is recommended to review the manufacturer’s IFU (Instructions for Use) to determine what they have tested and validated for their probe.
What do the experts and authorities say about the importance of cleaning?
It is recognized that only a properly cleaned probe can allow for an optimal disinfection process and the American Institute for Ultrasound in Medicine (AIUM) states that "adequate transducer preparation is mandatory."1 If you skip the cleaning step or perform it inadequately, you will compromise the entire disinfection process.
When we review the Centers for Disease Control (CDC) website, they define cleaning as:
"Cleaning is the removal of foreign material (e.g., soil, and organic material) from objects and is normally accomplished using water with detergents or enzymatic products. Thorough cleaning is required before high-level disinfection and sterilization because inorganic and organic materials that remain on the surfaces of instruments interfere with the effectiveness of these processes."2
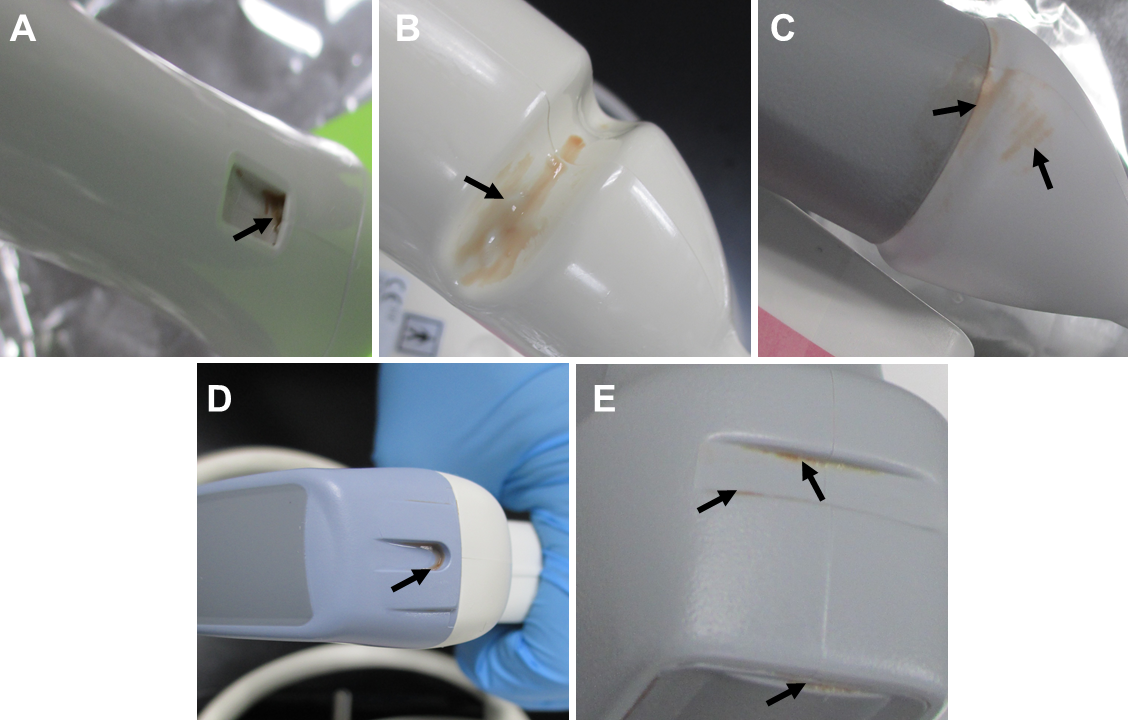
In the review of AIUM's recommendations, cleaning of the transducer is emphasized. They highlight just how crucial it is to clean your probes prior to high-level disinfection. Cleaning and disinfection are required even if a probe cover or sheath is used:
Meticulous cleaning of the instrument is the key to an initial reduction of the microbial/organic load by at least 99%. This cleaning is followed by a disinfecting procedure to ensure a high degree of protection from infectious disease transmission, even if a disposable barrier covers the instrument during use.
All this sounds very manual and could be riddled with the potential for human error. Let’s keep looking at the process and what could be the outcome if an ultrasound probe is not properly cleaned prior to high-level disinfection. Why is cleaning so necessary if you're going to high-level disinfect the transducer?
Cleaning is necessary because an inadequately or improperly cleaned endocavity probe, like a transvaginal or transrectal probe, can result in either bioburden and/or biofilm beginning to form.
Bioburden is the “degree of microbial contamination or microbial load; the number of microorganisms contaminating an object."4 Bioburden includes both microscopic debris and debris that is visible to the naked eye and refers to tissue, body fluids, bacteria, or any other biologic material present on, or in, an instrument or device after use on, or in, a patient. Varying degrees of bioburden will be present on an object after use on a patient; the accumulation of bioburden on used equipment is unavoidable. Once bioburden is present on a surface, biofilm formation is not far behind.5
Biofilm is “a slime-enclosed community of bacterial colonies that is very difficult to eradicate even with the most powerful antibiotics or sterilizing systems. Biofilms can occur on any body surface, on teeth (as dental plaque), medical equipment, medical tubing, contact lenses and elsewhere. They are held together by a matrix produced by the bacteria themselves and within this the bacteria communicate by chemical messengers, and generate proteins including enzymes that inactivate some antibiotics”.) 6 Biofilm attaches themselves to the surface of the ultrasound probe and develops a barrier, making them highly resistant to disinfection and removal. Organic material absorbs germicidal molecules and inactivates them.7
Biofilm can accumulate and harden on a probe. Biofilm can interfere with the antimicrobial activity of disinfectants in at least two ways:
- Leftover cleaning residual (organic or inorganic matter) left on the probe decreases the margin of safety and lengthens the exposure time required to kill the entire microbial load.8
- Any remaining transducer gel can also act as a barrier to the high-level disinfectant and diminish the efficacy of reusable disinfectant.8
Manual cleaning errors; could this process be something that automation could solve? Automation of the process would result in a reduction in human error and create a repeatable and reliable outcome.By automating the process, it would remove transducer gel, tissue, body fluids, bacterial, and other biological material, that could be present on the soiled ultrasound probe.A validated process cycle could accomplish this important step in the reprocessing of ultrasound probes every time. The same cleaning process done the same way each and every time with the incorporation of automation.
kNOw RISK is something each healthcare institution should constantly review as failures in the reprocessing of intracavity probes open them up to significant risk in many areas. Institutional risks due to improper or failed processes and failure to adhere to reprocessing guidelines can have serious outcomes for both patients, staff, and the institution itself, such as:

However automated cleaning of ultrasound probes can and has been done! CS Medical has been designing, developing, and manufacturing ultrasound probe cleaning and high-level disinfection solutions since 2003. Our latest US FDA cleared medical device is Ethos, the first cleaner disinfector of endocavity and surface ultrasound probes. Ethos removes the manual aspects of both the cleaning and high-level disinfection of transvaginal, transrectal, and surface ultrasound probes that are classified as semi-critical instruments within the Spaulding Classification.To learn more about Ethos automated cleaner disinfector visit our website at www.csmedicalllc.com/ethos or contact our team at 877-255-9472. CS Medical is setting the standard for reprocessing intracavity ultrasound probes. Ethos is the New Era in endocavity ultrasound probe reprocessing. Go with automation and reduce risk to patient, staff, and probe.
References:
- AIUM Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Transducers Between Patients & Safe Handling and Use of Ultrasound Coupling Gel, https://www.aium.org/resources/official-statements/view/guidelines-for-cleaning-and-preparing-external--and-internal-use-ultrasound-transducers-and-equipment-between-patients-as-well-as-safe-handling-and-use-of-ultrasound-coupling-gel
- CDC Guideline for Disinfection and Sterilization in Healthcare
Facilities, 2008.
https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf - Abujudeh, Hani H and Michael A Bruno. Radiology Noninterpretive Skills: The Requisites eBook. Elsevier Health Sciences, 2017. (Page 179)
- "Bioburden." Farlex Partner Medical Dictionary; 2012 [accessed 2023 May 25]. [1 p]. Available: http://medicaldictionary.thefreedictionary.com/bioburden.
- James Davis, Retained Bioburden On Surgical Instruments After Reprocessing: Are We Just Scraping the Surface? http://patientsafety.pa.gov/ADVISORIES/documents/201706_71.pdf
- Biofilm. (n.d.) Collins Dictionary of Medicine. (2004, 2005). Retrieved May 24 2023 from https://medical-dictionary.thefreedictionary.com/biofilm
- Growing Patient Safety Concern: Improperly Sterilized High-Level Disinfection (HLD) Equipment The Joint Commission. Available at: https://www.jointcommission.org/
- Tammy Tunstall, Journal of Diagnostic Medical Sonography, Infection Control in the Sonography Department. https://journals.sagepub.com/doi/pdf/10.1177/8756479310374362
