The Science Behind Spaulding Classification
Medical devices and other items in patient care areas must be cleaned and disinfected to prevent the spread of microorganisms and healthcare-associated infections (HAIs). Different devices, surfaces, and patient care related items pose different levels of risk for causing HAIs. The Spaulding classification was developed by Earle Spaulding in 1939 to create a standardized method for classifying the level of risk for each medical device (1). The Spaulding classification for determining infection risk associated with medical devices has been used by multiple regulatory agencies as a rational, standardized approach to determine the levels of disinfection required for medical devices in healthcare settings. Here we will discuss the science behind how each Spaulding classification corresponds to the level of disinfection or sterilization required, and the differences between each of these disinfection procedures from a regulatory and microbiological standpoint.
The Spaulding Classification for Medical Devices
The Spaulding classification divides medical devices into three classes: non-critical, semi-critical, and critical based upon the risk a device poses in causing HAIs (2). Non-critical medical devices are devices used on intact skin and pose the least risk of causing HAIs (2). Semi-critical medical devices, which pose a moderate relative risk for infection, come in contact with mucous membranes or non-intact skin (2). The devices with the highest risk of causing an infection are critical devices, which are defined as devices that come in contact with sterile body sites such as the bloodstream, heart, and brain (2). It is important to note that a particular device or device type could fall under multiple Spaulding classifications depending upon its use. A transabdominal ultrasound probe may be considered non-critical when used on intact skin, but would be considered semi-critical when used on a visible skin lesion.
Medical Devices Should Be Reprocessed According to their Spaulding Classification
| Spaulding classification | Definition | Examples | Recommended reprocessing | Examples of disinfectant/sterilant1 |
|---|---|---|---|---|
| Non-critical | Devices used on intact skin | Stethoscopes, blood pressure cuffs, transabdominal ultrasound probes* | Cleaning and low- or intermediate- level disinfection | Alcohols, Iodophor, Phenolics, Household bleach, Quaternary ammonium-based disinfectant2 |
| Semi-critical | Devices that come in contact with mucous membranes or non-intact skin | Transvaginal and transrectal ultrasound probes, Transesophageal echocardiogram probes | Cleaning and high-level disinfection | Glutaraldehyde, Ortho-pthaldehyde, Peracetic acid, Hydrogen peroxide, Hypochlorite |
| Critical | Devices that come in contact with sterile body sites | Surgical instruments (scalpels, forceps etc.), implanted devices | Cleaning and sterilization | Steam, Ethylene oxide, Hydrogen peroxide gas plasma, peracetic acid, hydrogen peroxide |
1Some high level disinfectants are also considered sterilants when used for longer contact times/increased temperature/concentration etc.
2Considered a low-level disinfectant
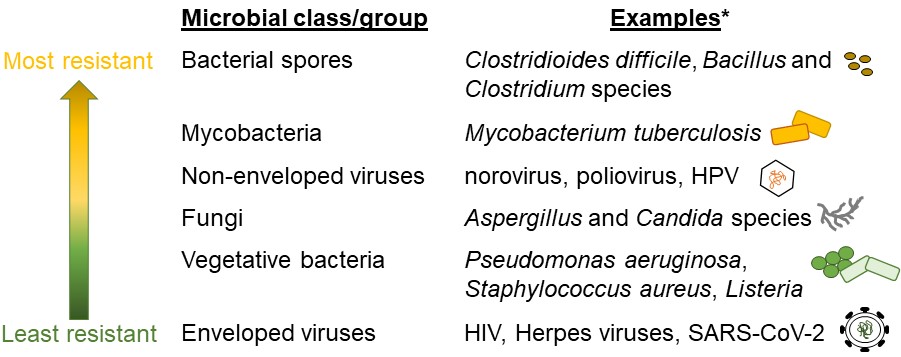
Low-level disinfection kills some vegetative bacteria, fungi, and viruses. Vegetative bacteria are live, actively growing bacteria (4) and are easier to kill than dormant bacterial spores (Figure 1). Low-level disinfection procedures do not eliminate more difficult to kill microorganisms, such as non-enveloped viruses like human papillomavirus (HPV) and poliovirus, mycobacteria, and bacterial spores. Therefore, these and other microorganisms that are similarly resistant to low-level disinfection can remain on probes following reprocessing. As the term implies, intermediate disinfection procedures kill a larger variety of microorganisms than low-level disinfection including all vegetative bacteria and most fungi and viruses. Some intermediate-level disinfectants may also kill mycobacteria at low numbers. However, intermediate disinfection is ineffective for killing bacterial spores. Despite the potential for some microorganisms to remain on low- or intermediate-level disinfected non-critical devices, there is a relative low risk for infection. Intact skin provides an excellent barrier to infection with microorganisms, particularly to lower numbers of microorganisms, because it is exposed to environmental organisms daily. Further, millions of microorganisms live on the skin naturally and can help defend against pathogenic or disease-causing microorganisms (5). Therefore, it is unlikely that microorganisms introduced to intact skin from properly disinfected non-critical ultrasound probes will cause infection.
Medical devices considered semi-critical by the Spaulding classification system should undergo high-level disinfection according to CDC and FDA guidance and AAMI ST58 (2,3,6,7). High-level disinfection procedures kill all vegetative bacteria, mycobacteria, fungi, viruses, and small numbers of bacterial spores (2). Mucous membranes are home to a community of microorganisms that must be kept in check. Therefore, these sites are specially adapted to keep microorganisms from invading the tissues making mucous membranes fairly resistant to infection with small numbers of bacterial spores, but not as resistant to infection as intact skin.
Sterilization procedures should be used to reprocess critical medical devices according to multiple regulatory and professional societies (2,3,6,7). All living microorganisms including bacteria, fungi, viruses, and bacterial spores are killed by sterilization. Critical devices are used on sterile body sites that are highly susceptible to infection. The introduction of microorganisms into these normally sterile sites can lead to infection and/or an immune response that can cause irreversible tissue damage and even death.
The Importance of High-Level Disinfection for Semi-Critical Devices
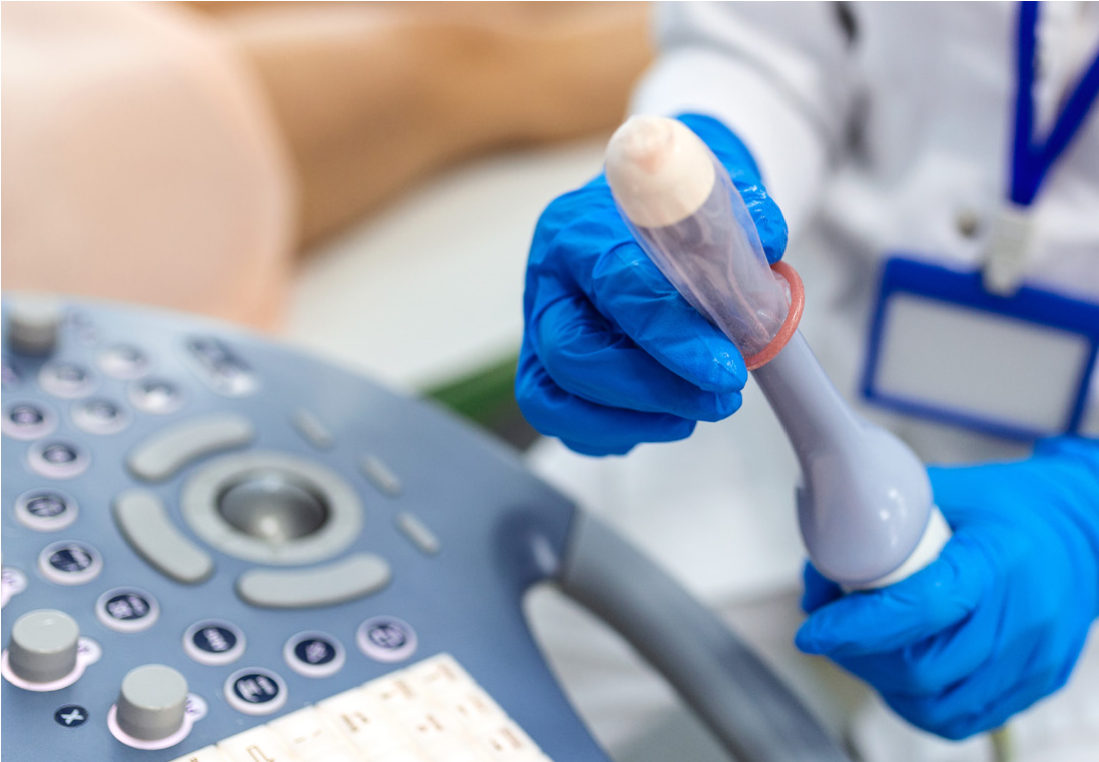
Many delicate devices such as ultrasound probes are not compatible with sterilization procedures and must instead be subjected to high-level disinfection and used with a sterile sheath (7). Sheath perforations are reported to occur 0-81% of the time, depending upon the manufacturer (8-11); placing more importance on ensuring ultrasound probes are properly cleaned and high-level disinfected to prevent HAIs. However, The Joint Commission recently stated that “performing intermediate- and high-level disinfection and sterilization of medical equipment, devices, and supplies” (IC.02.02.01, EP 2) was one of the top 5 requirements most frequently identified as “not compliant” in 2022 (12). Surveys of infection preventionists confirm The Joint Commission’s findings suggesting that non-compliance with recommended disinfection procedures for medical devices is widespread.
Many non-compliance issues identified in the surveys involved not using high-level disinfection on semi-critical ultrasound probes (13,14). Non-compliance with high-level disinfection tends to be higher than low- or intermediate-level disinfection because it is generally more time consuming. Further, many healthcare workers may not think that high-level disinfection is important or may not understand the distinction between low-, intermediate-, and high-level disinfection (13,15).
Devices subjected to low- and intermediate-level disinfection could remain contaminated with some microorganisms. The level of noncompliance with reprocessing standards is alarming, particularly when semi-critical ultrasound probes are low- or intermediate-level disinfected. Because low-level and intermediate disinfection do not necessarily kill all microorganisms, probes that are subjected to these disinfection procedures can remain contaminated with certain microorganisms (16). Many of the microorganisms remaining on these probes are inherently more resistant to disinfection. Transvaginal probes subjected to low-level disinfection were found to remain contaminated with HPV and C. trachomatis DNA following disinfection (17). However, DNA is not necessarily indicative of infectious microorganisms. Further, quaternary ammonium compounds, a common class of low-level disinfectant found in hospitals, are ineffective against Mycoplasma, including Mycoplasma genitalium (18). Therefore, proper cleaning and high-level disinfection of semi-critical transvaginal ultrasound probes is critical to prevent HAIs.
In addition to killing bacteria and viruses that are inherently resistant to disinfection, high-level disinfection also has the advantage of killing low levels of bacterial spores. C. difficile is a common environmental bacterium and one of the most common causes of HAI in the United States (19). Healthy people can harbor C. difficile in their gastrointestinal tract(20) leading to C. difficile contamination of transrectal probes, even when used on seemingly healthy patients(21). Hospital-acquired C. difficile infection from use of a contaminated transrectal probe is unlikely, given that C. difficile grows in the large intestine (20), a site that is “upstream” of any tissues expected to come in contact with transrectal probe. However, wiping contaminated surfaces with non-sporicidal disinfectant wipes has been shown to contaminate C. difficile-free surfaces (22,23). It seems likely that a similar situation could occur with contaminated ultrasound probes, where storage of improperly disinfected probes contaminated with C. difficile could lead to environmental contamination. Further, detergent and disinfectant wipes retain C. difficile spores(22-25) that can persist in the environment for months if not subjected to an effective disinfectant (26). Environmental C. difficile contamination is difficult to address and is known to lead to hospital-acquired C. difficile infection (27). Using a high-level disinfectant procedure that can kill small numbers of spores and/or wash them away could help prevent this environmental contamination and kill spores that are left on transrectal probes, wipes, and reprocessed devices.
| Number of bacteria | Log Reduction* (Log10) | Percent Reduction* (%) |
|---|---|---|
| 1000000 | 0 | 0 |
| 100000 | 1 | 90 |
| 10000 | 2 | 99 |
| 1000 | 3 | 99.9 |
| 100 | 4 | 99.99 |
| 10 | 5 | 99.999 |
| 1 | 6 | 99.9999 |
High-level disinfection provides a larger margin of safety. All disinfectants used to reprocess medical devices must be tested by device manufacturers prior to use on their devices in a clinical setting. Manufacturers must demonstrate that a particular disinfectant and/or disinfection process can kill a variety of microbes, when used according to the manufacturer’s instructions. Disinfectants or disinfection processes are then classified as low-, intermediate-, or high-level based upon their ability to kill certain representative bacteria. To achieve low-level disinfection of medical devices, according to the FDA, manufacturers must test and demonstrate, at a minimum, that the disinfectant and/or disinfection procedure can kill at least 6 log10 of E. coli, S. aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa (28) on contaminated devices. These clinically-relevant bacteria are relatively easy to kill and could be considered to be representative of most vegetative bacteria. To demonstrate intermediate disinfection, a disinfectant must meet the standards of a low-level disinfectant and kill at least 3 log10 of a representative Mycobacterium species such as Mycobacterium terrae on contaminated devices (28,29). High-level disinfectants must be able to kill 6 log10 of a Mycobacterium species (M. terrae) on devices and undergo additional testing to show killing of vegetative bacteria, fungi, and some bacterial spores (29). Sterilization procedures must pass the highest bar and demonstrate killing of 6 log10 spores on contaminated devices within the manufacturer’s recommended contact time (29). Chemical sterilants must also meet the requirements of a high-level disinfectant in addition to passing further viricidal testing.
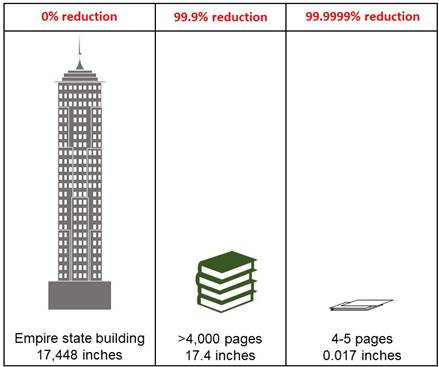
Examination of the standards expected for intermediate- and high-level disinfection by the FDA could lead even experienced healthcare professionals to question the need for high-level disinfection for semi-critical devices. The major difference between intermediate- and high-level disinfection standards is the ability to kill at least 3 log10 or 6 log10 of Mycobacterium, respectively. Log10 reductions in bacterial populations are not linear and may be more easily expressed as percentages, where a 99.9% reduction represents a 3 log10 reduction, while a 99.9999% reduction represents a 6 log10 reduction (Table 2). The extra 0.0999% reduction afforded by high-level disinfection may not seem like a lot, but it results in magnitudes of difference. For example, the Empire State Building is approximately 1,454 feet or 17,448 inches tall (Figure 2). If the height of the Empire State Building was reduced by 99.9999%, it would be the height of a stack of 4-5 pieces of normal copy paper. In contrast, if the height of the Empire State Building was only reduced by 99.9%, it would be approximately 17.4 inches tall. This is still a large size reduction, but consider that it would require over 4,000 pieces of paper to create a stack 17.4 inches high (for reference, the Oxford English dictionary is a little over 1000 pages long).

The expectation for high-level disinfection to kill large numbers of mycobacteria is not necessarily rooted in the idea that medical devices will become contaminated with high numbers of mycobacteria during routine procedures. Rather, Mycobacterium killing is used as a marker for bacteria that are very resistant to killing with disinfectants. Mycobacteria are coated in a thick layer of lipids in addition to the protective layers typically found on bacteria. This extra lipid layer makes it difficult for disinfectants to enter into and kill the bacteria. Using the hierarchical model shown in Table 1, a high-level disinfectant that can kill mycobacteria, is likely to kill other microorganisms that are less or similarly resistant to disinfection. Taking this logic one step further, the effectiveness of high-level disinfectants against large numbers of mycobacteria could be expected to provide a larger margin of safety in the removal of all microorganisms (except small numbers of bacterial spores) than intermediate disinfectants, even those effective against small numbers of mycobacteria and/or non-enveloped viruses. Therefore, high-level disinfection can provide increased confidence that all microorganisms on a contaminated device are killed (except small numbers of bacterial spores) to prevent HAIs.
The prevention of HAIs from contaminated medical devices relies on using an appropriate cleaning and disinfection protocol. Over 60 years ago, Spaulding proposed a standardized approach to classify medical devices according to their use and risk to patients. Though small changes have been made to accommodate medical and technological advancements, the basic principles underlying Spaulding’s classification system are still relevant today, given our current understanding of infection prevention, physiology, and microbiology. Thus, Spaulding’s classification of medical devices remains an important tool for regulatory agencies and healthcare professionals to guide medical device reprocessing, protect patients, and prevent HAIs.
References:
- Medical, C., FAQ: What is Spaulding Classification?, in Probing Questions. 2021.
- Rutala, W.A., D.J. Weber, and Healthcare Infection Control Practices Advisory Committee, Guideline for Disinfection and Sterilization in Healthcare Facilities, Centers for Disease Control and Prevention. 2008. p. 14-21.
- Society of Diagnostic Medical Sonography, Sonographer Best Practices for Infection Prevention and Control: Reprocessing the Ultrasound Transducer. 2022: Plano, TX.
- Bruslind, L., General Microbiology. 2019: Oregon State University.
- Byrd, A.L., Y. Belkaid, and J.A. Segre, The human skin microbiome. Nat Rev Microbiol, 2018. 16(3): p. 143-155.
- AAMI Chemical Sterilants Hospital Practices Working Group, ANSI/AAMI ST58:2013 (R2018) Chemical Sterilization And High-Level Disinfection In Health Care Facilities. 2018, Association for the Advancement of Medical Instrumentation: Arlington, VA.
- Food and Drug Administration, Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling, Centers for Devices and Radiological Health. Health, Editor. 2017.
- Basseal, J.M., S.C. Westerway, and J.A. Hyett, Analysis of the integrity of ultrasound probe covers used for transvaginal examinations. Infect Dis Health, 2020. 25(2): p. 77-81.
- Hignett, M. and P. Claman, High rates of perforation are found in endovaginal ultrasound probe covers before and after oocyte retrieval for in vitro fertilization-embryo transfer. J Assist Reprod Genet, 1995. 12(9): p. 606-9.
- Milki, A.A. and J.D. Fisch, Vaginal ultrasound probe cover leakage: implications for patient care. Fertil Steril, 1998. 69(3): p. 409-11.
- Pugliese, G. and M.S. Favero, Condoms as Probe Covers for Transvaginal Sonography.
- The Joint Commission, Top 5 Most Challenging Requirements for 2022. April 19, 2023. The Joint Commission Online.
- Carrico, R.M., S. Furmanek, and C. English, Ultrasound probe use and reprocessing: Results from a national survey among U.S. infection preventionists. American Journal of Infection Control, 2018. 46(8): p. 913-920.
- Nyhsen, C.M., et al., Infection prevention and ultrasound probe decontamination practices in Europe: a survey of the European Society of Radiology. Insights Imaging, 2016. 7(6): p. 841-847.
- Westerway, S.C. and J.M. Basseal, The ultrasound unit and infection control - Are we on the right track? Ultrasound, 2017. 25(1): p. 53-57.
- Leroy, S., Infectious risk of endovaginal and transrectal ultrasonography: systematic review and meta-analysis. J Hosp Infect, 2013. 83(2): p. 99-106.
- Casalegno, J.S., et al., High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PLoS One, 2012. 7(10): p. e48137.
- Eterpi, M., G. McDonnell, and V. Thomas, Decontamination efficacy against Mycoplasma. Lett Appl Microbiol, 2011. 52(2): p. 150-5.
- Healthcare-Associated Infections Community Interface, Clostridioides difficile Infection (CDI) Tracking. 2022; Available from: https://www.cdc.gov/hai/eip/cdiff-tracking.html.
- Schäffler, H. and A. Breitrück, Clostridium difficile - From Colonization to Infection. Front Microbiol, 2018. 9: p. 646.
- Australian Society for Ultrasound Medicine, Guidelines for Reprocessing Ultrasound Transducers. Australas J Ultrasound Med, 2017. 20(1): p. 30-40.
- Nkemngong, C.A., et al., Disinfectant wipes transfer Clostridioides difficile spores from contaminated surfaces to uncontaminated surfaces during the disinfection process. Antimicrob Resist Infect Control, 2020. 9(1): p. 176.
- Siani, H., C. Cooper, and J.Y. Maillard, Efficacy of “sporicidal” wipes against Clostridium difficile. Am J Infect Control, 2011. 39(3): p. 212-8.
- Kenters, N., et al., Effectiveness of various cleaning and disinfectant products on. Antimicrob Resist Infect Control, 2017. 6: p. 54.
- Ramm, L., et al., Pathogen transfer and high variability in pathogen removal by detergent wipes. Am J Infect Control, 2015. 43(7): p. 724-8.
- Fekety, R., et al., Epidemiology of antibiotic-associated colitis; isolation of Clostridium difficile from the hospital environment. Am J Med, 1981. 70(4): p. 906-8.
- Center for Disease Control and Prevention, FAQs for Clinicians about C. diff. 2022; Available from: https://www.cdc.gov/cdiff/clinicians/faq.html.
- Food and Drug Administration, Medical Washers and Medical Washer-Disinfectors - Class II Special Controls Guidance Document for the Medical Device Industry and FDA Review Staff, Centers for Devices and Radiological Health. 2002.
- AAMI Instructions for Reusable Device Reprocessing Working Group, AAMI TIR12:2020 Designing, Testing, And Labeling Medical Devices Intended For Processing By Health Care Facilities: A Guide For Device Manufacturers, Association for the Advancement of Medical Instrumentation. 2020.