Clean Probes are Critical for a Successful High-Level Disinfection.
A Comparison between the Cleanliness of Manually-cleaned Endocavity and surface ultrasound Probes to those Reprocessed with CS Medical Ethos® Automated Cleaner Disinfector
Reusable medical devices including ultrasound transducers or probes must be cleaned and subsequently disinfected between each patient to prevent the spread of infection. Proper cleaning of ultrasound probes is essential to promoting patient safety, keeping probes functioning correctly, and preventing healthcare acquired infections (HAIs). Cleaning is the process of removing debris and foreign material from an ultrasound probe and is a crucial step of the high-level disinfection process. Ultrasound probes can come in contact with multiple non-sterile patient tissues leaving the probes contaminated with microorganisms. The cleaning process can remove 4-6 log10 bacteria from contaminated medical devices (1-4). Reductions in bacterial numbers on contaminated devices promotes bacterial killing during subsequent high-level disinfection; due to lower numbers of microorganisms being easier to kill with disinfectants, when compared to higher concentrations. The physical removal of debris is also important to ensure proper high-level disinfection because soil and debris can inhibit or inactivate some disinfectants (5-6) and/or protect microorganisms from being killed by the disinfectant (2, 7, 8). Inadequate bacterial killing on ultrasound probes can promote the formation of biofilms or groups of microorganisms that live together and are protected by an extracellular matrix. Bacterial biofilms are more difficult to remove with cleaning and are more resistant to killing by disinfectants than free-living bacteria. Thus, the need for proper cleaning prior to disinfection is instrumental in providing safe, disinfected probes for use in clinical procedures.
Traditionally, cleaning of ultrasound probes is completed using manual processes that generally involves wiping with an impregnated wipe, cloth or submerging the probe in large bins with detergent and water. Currently, there is no standardized method for manual cleaning procedures. Instead, manufacturers are required by the FDA to provide cleaning procedures and a list of detergents and disinfectants that have been tested and approved to clean each probe. Manual cleaning processes can vary widely based upon probe type and manufacturer. For example, the intracavity transducer care document for FUJIFILM SonoSite probes states to “…wipe the transducer from the cable to the scanhead, using an approved pre-moistened wipe or a cloth dampened with an approved cleaner” (9). In contrast, the care cards provided by BK medical® (10) and Canon (11) provide procedures for cleaning probes using wiping and immersion processes. Further, BK medical’s cleaning procedure states “Use a soft, non-abrasive brush (for example, a surgical nail brush) to thoroughly clean all parts of the transducer…”, but the Canon cleaning procedure states “Do not use a brush, because it may damage the transducer.” Each probe must be cleaned in accordance with the manufacturers’ cleaning procedures to ensure the probes are clean prior to disinfection. However, variability between manufacturers’ procedures could easily lead to skipped steps or confusion about how each probe should be cleaned, particularly for newer staff members who may be less familiar with the subtle differences between each cleaning procedure. Automated cleaning such as that provided by the CS Medical Ethos® Automated Cleaner Disinfector can eliminate the variability in manual cleaning procedures and provides a straightforward workflow to clean and disinfect multiple probe models from different manufacturers.
| Endocavity probes | Surface probes |
|---|---|
| 1. GE BE9CS-RS | 10. GE 4CD |
| 2. GE IC5-9-D | 11. Philips C5-1 |
| 3. Toshiba PVM-651VT | 12. Siemens Acuson 10L4 |
| 4. Philips C8-4V | 13. Siemens Acuson 10L4 |
| 5. Siemens Acuson 10MC3 | |
| 6. Philips C10-3v | |
| 7. Siemens Acuson 10MC3 | |
| 8. Philips C10-3v | |
| 9. Siemens Acuson 10MC3 |
In addition to being highly variable between manufacturers, manual cleaning processes can be tedious, particularly with more complex probes. With the CS Medical Ethos® Automated Cleaner Disinfector cleaning and high-level disinfection of ultrasound probes is completely automated using a single run. Automated cleaning prevents staff from tediously wiping down crevices and provides hands-off time for other tasks to be completed. The warm water pre-rinse in the Ethos® helps dissolve and remove water soluble ultrasound gel from difficult to reach crevices on probes. Any gel remaining on a probe following cleaning could impact the efficacy of subsequent high-level disinfection. While the Ethos® Automated Cleaner Disinfector can provide extra time for staff and promote the removal of ultrasound gel, we sought to test if the Ethos® cleans probes similar to manual cleaning procedures used by trained professionals.
Probes cleaned by manual and automated methods were sampled for remaining residue
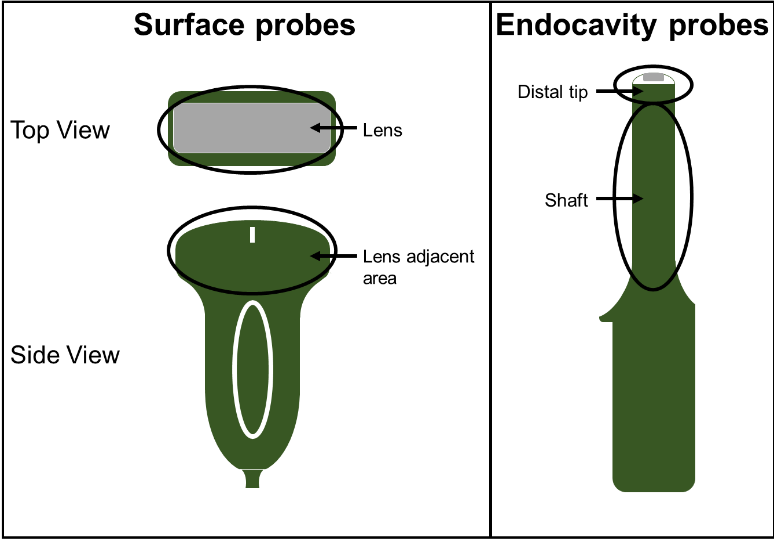
Three experienced clinicians were recruited to manually clean common endocavity and surface ultrasound probes. Each clinician cleaned 13 probes encompassing 9 different probe models. One probe model was cleaned in triplicate and two models were cleaned in duplicate as shown in Table 1.
Prior to cleaning, the surface probes were soiled on the lens and lens adjacent areas (Figure 1), while the endocavity probes were soiled on the distal tip and shaft areas. A model soil (12) comprised of 75% Edinburgh Soil/25% ultrasound gel (w/w) was applied directly onto each probe surface without the use of a protective sheath and allowed to dry. This soiling method provided the “worst-case scenario” cleaning challenge. The clinicians were provided with the same cleaning wipes used at their clinics and any other available supplies they requested during the cleaning process. Following cleaning by experienced clinicians, CS Medical staff carefully inspected each probe for remaining visible soil. The cleaned probes were then swabbed in their entirety to collect any remaining residue on the probes. The residue was assayed for protein as a soil marker based upon FDA guidance for reprocessing of reusable medical devices (13).
In addition to manual cleaning, probes were cleaned in the Ethos® automated cleaner disinfector. Probeswere soiled as before and run through one complete cycle in the Ethos® with the manufacturer’s recommended amount of AquaCideProbes cleaned in the Ethos® were swabbed and assayed for protein in the same manner as manually cleaned probes.
Manually cleaned probes remained contaminated with visible soil
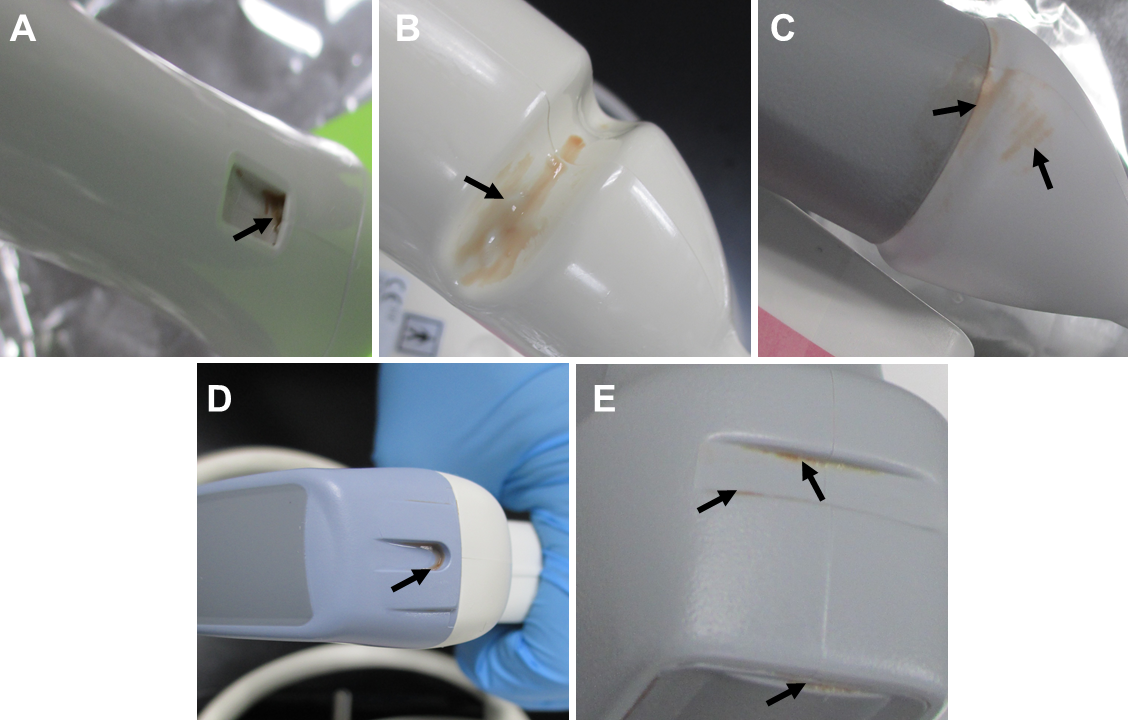
Examination of the manually cleaned probes revealed visible soil remaining on many of the probes. Residual soil was often observed within indentations on the probes that are used for accessories, though no accessories were used in this study (Figure 2). All of the experienced clinicians used supplemental techniques to clean difficult areas of the soiled probes including indentations. These supplemental techniques included using dry swabs or swabs dipped in water to remove soil, or wrapping a wipe around the swab before applying it to the soiled areas. However, even with the use of supplemental procedures, visible soil remained within indentations on many of the probes after manual cleaning suggesting these areas are difficult to clean using manual methods. Clinician 1 acknowledged the presence of remaining soil in a divot on one of the probes and stated that in the clinic the probe would be run through a disinfection cycle and visually inspected again. If there was remaining soil on the probe following disinfection, the probe would be cleaned and disinfected again, until no visible soil remained. The soil remaining in this divot following cleaning by clinician 1 was excluded from the protein analysis because the tools required to complete the cleaning to the clinician’s satisfaction (i.e. a method for completing a disinfection cycle) were not available.
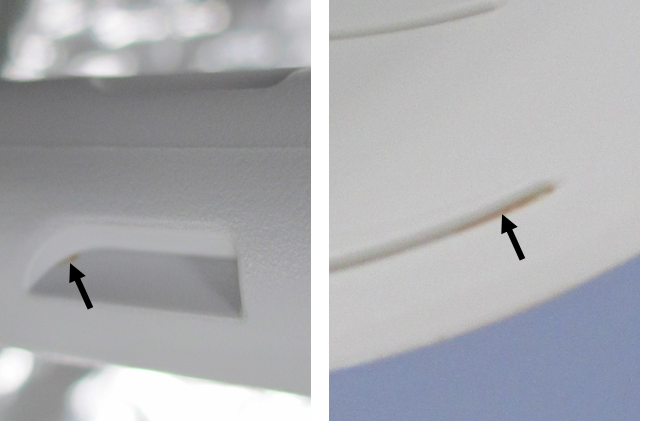
Visible soil remaining on the probes following cleaning was not acknowledged for any other probe or by the other clinicians. The level of soil remaining on probes varied considerably, but was often a visibly small amount. In some cases, the amount of visible remaining soil on the probe combined with its location (i.e. in a crevice/crack) made it difficult to see with the unaided eye (Figure 3). As a clinician’s day gets busy cleaning gets rushed so they can turn the room around for another patient, which can also cause improper cleaning.
| Operator | Clinician 1 | Clinician 2 | Clinician 3 |
|---|---|---|---|
| Total protein (μg) | 660 | 335 | 220 |
Following visual inspection, the amounts of proteinaceous residue remaining on each probe was quantified. Quantifiable amounts of residue were detected on 38 out of 39 probes after manual cleaning. The total amount of residue remaining on all 13 probes after cleaning was operator dependent and ranged from 220 µg to 660 µg (Table 2). These data suggest that manual cleaning procedures and effectiveness are operator dependent.
Manually cleaned probes remained contaminated with visible soil
Visual examination of the probes following a complete run in the Ethos® showed no visible soil on any of the cleaned probes, including within the indentations that proved difficult to clean by manual methods. Further, quantifiable protein residue was below the lower limit of quantification for all probes listed in Table 1, when cleaned using the Ethos®. In contrast, manual cleaning reduced protein residue below the lower limit of detection for only 3% of probes in this study. Together, these results show that the Ethos® Automated Cleaner Disinfector cleaned probes more consistently than manual methods.
Our study suggests there are two potential downfalls of manual cleaning methods: they can be highly operator dependent and divots in probes can be difficult to clean. The clinicians recruited for this study were experienced, with one clinician having 21 years of experience. However, differences in training or human error could result in the variability in manual cleaning between clinicians we observed. Recent guidance published by the Society of Diagnostic Medical Sonography (SDMS) recommends that cleaning of ultrasounds transducers should remove all visible gel, soil, and bioburden from probes including from indentations (14). In this study, manual cleaning with wipes resulted in visible soil on at least one probe cleaned by each of the three clinicians; most of which was found within probe divots. These observations suggest that meeting the recommended cleaning standards using manual methods may be more difficult than is appreciated. The clinicians tried to use supplemental techniques to clean these areas, but these techniques did not necessarily result in a cleaner probe and required clinicians to spend more time cleaning each probe. Some of the probes had very small amounts of visible soil that were difficult to see at first glance. These lightly soiled areas could easily be missed if a clinician is under time constraints or lighting in the reprocessing area is poor. Soil remaining on probes after cleaning can protect microbes from disinfection (2, 5-8) resulting in potentially contaminated probes following reprocessing.
Automated cleaning using the Ethos® Automated Cleaner Disinfector can alleviate many of the downfalls of manual cleaning. In our study, the Ethos® consistently cleaned probes from three different probe manufacturers using one automated workflow, which can help eliminate operator dependent cleaning and confusion or errors due to differences in manual cleaning processes between manufacturers. Automated cleaning by the Ethos® removed all visible soil in a single run, even in divots that were difficult to reach using manual methods resulting in probes cleaned to the standards put forth by industry leaders. Further, the automated nature of cleaning in the Ethos can eliminate the need for supplemental cleaning techniques and provide more hand-off time for staff to complete other clinical duties or documentation to help keep clinical departments running efficiently. In summary, the Ethos® Automated Cleaner Disinfector uses one streamlined and automated workflow to provide consistently clean endocavity and surface ultrasound probes to help prevent infections and meet patient safety standards. Automation of cleaning and high-level disinfection processes only furthers the necessary process improvements required to minimize hospital acquired infections.
References:
- Alfa, M.J., P. Degagne, and N. Olson. "Worst-case soiling levels for patient-used flexible endoscopes before and after cleaning." American Journal of Infection Control, 1999. 27(5): p. 392-401.
- Rutala, W.A., D.J. Weber, and H.I.C.P.A. Committee. "Guideline for Disinfection and Sterilization in Healthcare Facilities", C.f.D.C.a. Prevention, Editor. 2008. p. 14-21.
- Chu, N.S., D. McAlister, and P.A. Antonoplos. "Natural bioburden levels detected on flexible gastrointestinal endoscopes after clinical use and manual cleaning." Gastrointestinal Endoscopy, 1998. 48(2): p. 137-142.
- Hanson, P.J., et al. "Recovery of the human immunodeficiency virus from fibreoptic bronchoscopes." Thorax, 1991. 46(6): p. 410-412.
- Lambert, R.J. and M.D. Johnston. "The effect of interfering substances on the disinfection process: a mathematical model." J Appl Microbiol, 2001. 91(3): p. 548-55.
- Rutala, W.A. and D.J. Weber. "Uses of inorganic hypochlorite (bleach) in health-care facilities." Clin Microbiol Rev, 1997. 10(4): p. 597-610.
- Lewis, D.L. and M. Arens. "Resistance of microorganisms to disinfection in dental and medical devices." Nat Med, 1995. 1(9): p. 956-8.
- Muscarella, L.F. "Sterilizing dental equipment." Nature Medicine, 1995. 1(12): p. 1223-1224.
- FUJIFILM SonoSite. "Intracavity Transducer (ICT) Transducer Care Card". 2022: Bothell, WA.
- B.K. Medical. "Care and Cleaning Information for the BK Medical Product Range". 2021: Herlev, Denmark.
- Canon Medical Systems Corporation. "Guidelines for Cleaning, Disinfection, and Sterilization of Transducer". 2021.
- Alfa, M.J. and N. Olson. "Physical and composition characteristics of clinical secretions compared with test soils used for validation of flexible endoscope cleaning." Journal of Hospital Infection, 2016. 93(1): p. 83-88.
- Food and Drug Administration. "Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling", Center for Diagnostic and Radiological Health, 2018.
- Society of Diagnostic Medical Sonography. "Sonographer Best Practices for Infection Prevention and Control: Reprocessing the Ultrasound Transducer". 2022: Plano, TX.
