A Critical Element to Proper High-Level Disinfection
Pre-cleaning is essential, whether you high-level disinfect manually or with an automated system.
The use of automated disinfection devices does not eliminate this crucial step. If pre-cleaning is skipped or performed improperly, the disinfection procedure is compromised.
Cleaning is defined as "removal of visible soil (e.g. organic and inorganic material) from objects and surfaces and normally is accomplished manually or mechanically using water with deterg... ents or enzymatic products."
Why do you need to clean if you have an automated disinfection system?
Automated ultrasound disinfectors on today's market do not provide a cleaning cycle like their counterparts AER (Automated Endoscope Reprocessor). An ultrasound probe should be cleaned as cleaning removes inorganic and organic debris that can otherwise remain on the probe's surface and prevent the efficacy of the disinfection.
A well designed cleaning and disinfection process should answer the following questions:
- Is the facility and staff following manufacturer's instructions for cleaning or evidence-based guidelines?
- Are all staff visually inspecting the probes prior to high-level disinfection for residual soil and recleaning as needed before high-level disinfection?
- Does each staff member, with the responsibility of reprocessing ultrasound probes, have adequate training?
The American Institute for Ultrasound in Medicine (AIUM) recommends cleaning ultrasound transducers even if a probe cover or sheath is used:
"Meticulous cleaning of the instrument is the key to an initial reduction of the microbial/organic load by at least 99%. This cleaning is followed by a disinfecting procedure to ensure a high degree of protection from infectious disease transmission, even if a disposable barrier covers the instrument during use."

NUZyme™ Enzymatic Sponge being used to clean an ultrasound transducer prior to high-level disinfection
The cleaning process is extremely important as it removes inorganic and organic debris that can otherwise remain on the probe and defeat the efficacy of the disinfection. Cleaning incorporates both an enzymatic soak and a thorough rinsing of the enzyme from the ultrasound probe.
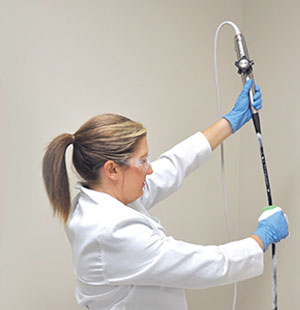
TEEZyme® TEE Probe Enzymatic Sponge being applied to the insertion tube of a TEE probe prior to high-level disinfection
Cleaning is one of the most difficult parts of dealing with medical equipment. It is the most critical reprocessing step, says Thomas Fischer, Ph.D., Chief Science Officer for CS Medical. "It removes more than 99.9 percent of the bioburden from the probe," he points out. "If a probe is not properly cleaned, terminal disinfection may not be possible. Failure to properly clean probes has considerable consequences for both the patient and the healthcare provider." Additionally, Dr. Fischer goes on to state, "If reprocessing is delayed, that allows for the accumulation and hardening of bioburden. Bio burden can dry into a nearly impervious substance - harder to remove than dried baby food on a high-chair."
Once you have cleaned the ultrasound transducer the next important step is to properly dry the probe. Drying is equally important as cleaning when it comes to automated ultrasound reprocessors. Any debris, gel or residual water can mitigate the disinfection process. When determining the proper drying method it is important to remember not to introduce new sources of contaminant that could affect the ability of the automated reprocessor to perform properly. A drying cloth should be low-lint so as not to leave debris on the probe. This debris can enter into the fluid flow path of the automated reprocessor and limit the flow of the high-level disinfectant thus resulting in an error or run abort by the reprocessor.
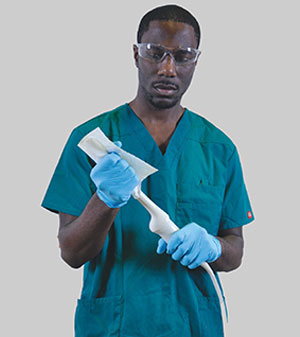
QwikDry® ultrasound drying cloth being used to dry an ultrasound transducer after high-level disinfection
A critical step that should not be overlooked at the conclusion of high-level disinfection is using a drying cloth that is free of contaminants. The cloth serves as another risk mitigation step and removes residual moisture that might exist from the automated reprocessor. Once again, the drying cloth should be low-lint and contaminant-free. A single-use, low-lint and gamma irradiated drying cloth is available from CS Medical. QwikDry® is a solution to remove moisture without reintroducing contaminants.
Conclusion
In conclusion, the first defense is a good offense and in healthcare, that means education. If employees are educated about how to clean and understand why certain steps are essential, they are much less likely to skip or shortchange those steps. In an environment that is very dynamic with new technologies, responsibilities, and turnover, ongoing education and training allows your staff to keep current.
References:
- The AIUM empowers ultrasound professionals to improve patient outcomes. https://www.aium.org
- CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf









