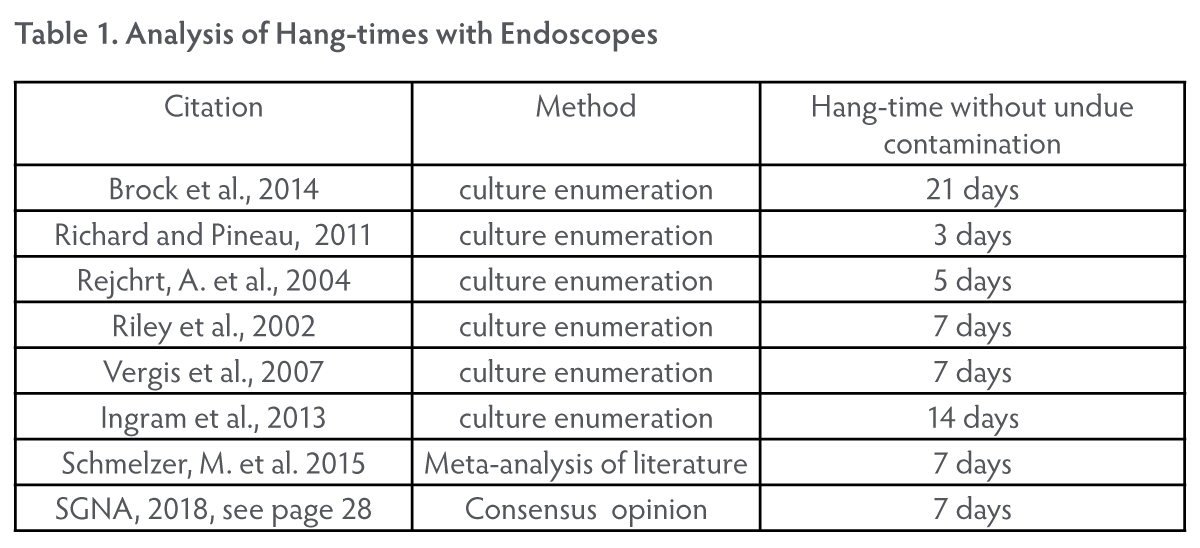
Study data supports seven (7) days hang time for TEE ultrasound probes in
cleanshield ultrasound probe storage cabinet between hld events models:
ac-te-03 and
ac-te-06 (110/220v)
CS Medical commissioned a study to determine how long a high-level disinfected TEE ultrasound probe can remain in an AirClean® Systems CleanShield® positive pressure HEPA filtered storage cabinet before needing to be reprocessed. The studies concluded that transesophageal echocardiogram (TEE) probes can be stored for at least seven days in the AirClean Systems CleanShield TEE Probe Storage Cabinet.
Detailed recommendations concerning permissible TEE probe hang times have not been made by governmental regulatory organizations. However, studies (see Table 1. “Analysis of Hang-times with ... Endoscopes”) have been performed with endoscopes (not TEE probes) in which hang-times of several days without “undue contamination” are documented. A review of these results by Schmelzer et al. (2015) and the Society of Gastroenterology Nurses and Associates (SGNA, 2018) point towards seven days as being an appropriate storage time given proper aseptic storage.
The approach used for evaluating the appropriateness of (7) seven-day hang times was to determine if the probe cabinet succeeded in minimizing microbial contamination in an environment that would normally result in excessive contamination. In this situation, “excessive contamination” is based on the European Standard EN16442:15 of 100 CFU/probe of non-pathogenic microorganisms (see section 6.5.3. page 19, CEN/TC 102, 2015). Siemens, GE, and Philips TEE probes (two of each type) were subjected to automated cleaning, disinfection, and rinsing. The probes were dried immediately upon removal, following probe manufacturer’s guidelines, with gamma-irradiated single-use drying cloths. Three probes (one of each type) were placed in a fully functional CleanShield TEE Probe Storage Cabinet, model AC-TE-03. The other set of TEE probes (also one of each type) were placed in an open positive control cabinet with similar configuration but without HEPA filtration. Both cabinets were placed in a room with moderate use by technical laboratory staff with ISO 8573 Class 4 air. This environment mimicked a well-trafficked clinical staging area. After one week the probes were sampled for bacterial contamination.
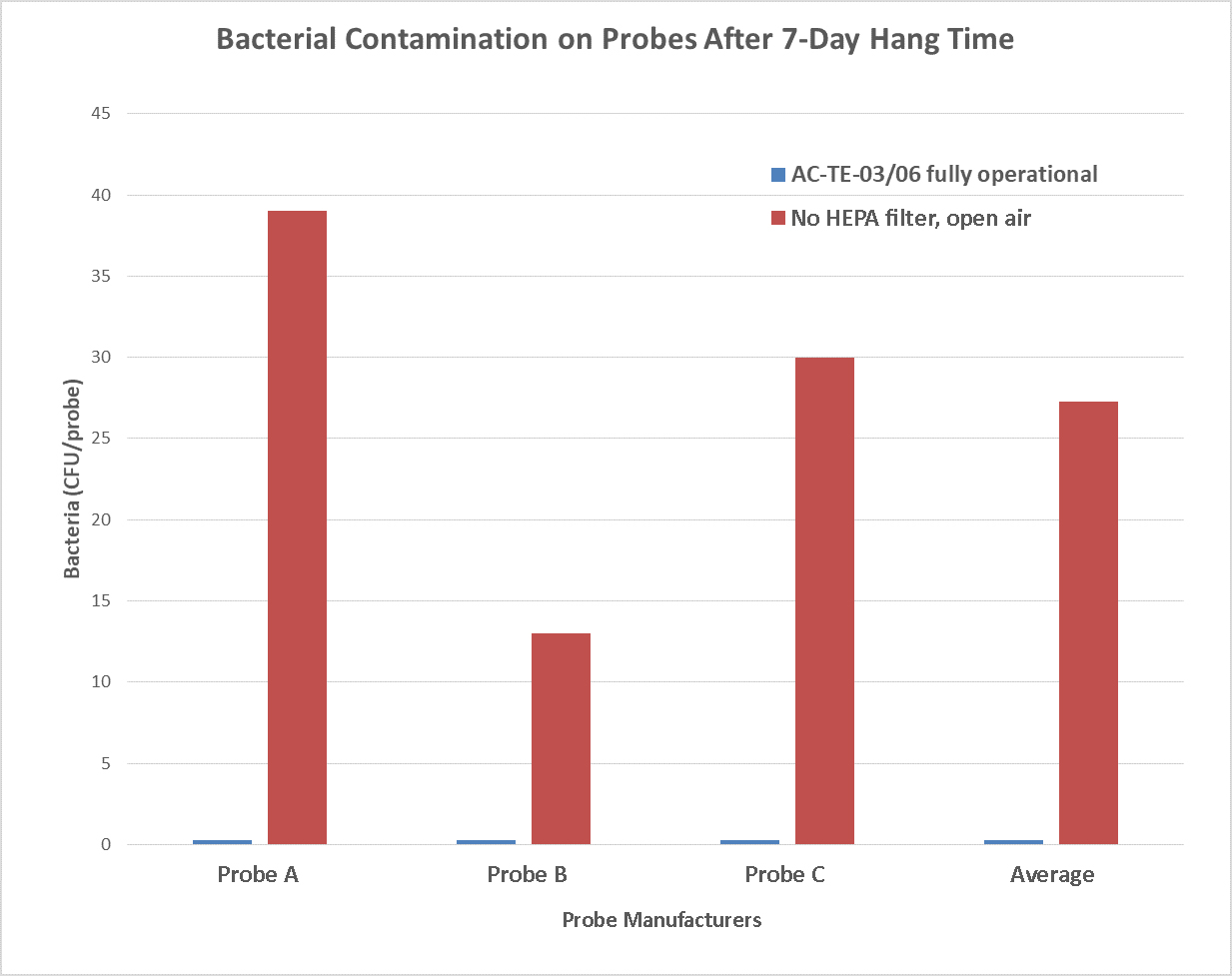
Conclusion
The TEE probes in the fully operational CleanShield AC-TE-03 cabinet remained measurably free of contamination over a 7 (seven) day period. In contrast, TEE probes that were stored without HEPA air filtration were heavily contaminated. The results in the control cabinet without HEPA filtration or door closure showed that each probe contacted 160 bacteria per 24 hour hang-period. These findings support the acceptability of hang-times of at least seven (7) days for TEE probes in the CleanShield TEE Probe Storage Cabinet, models AC-TE-03 and AC-TE-06 in either 110 or 220 volt configuration.
References
-
Brock, A., Steed, L., Freeman, J., Garry, B., Malpas, P. and Cotton, P. 2014. “Endoscope storage time: Assessment of microbial colonization up to 21 days after reprocessing.” Gastrointestinal Endoscopy 81(5): 1150-4.
-
Ingram, J., Gaines, P., Kite, R., Morgan, M., Spurling, S., and Winsett, R. 2013. “Evaluation of medically significant bacteria in conlonoscopes after 8 weeks of shelf life in open air storage.” Gastroenterology Nursing 36(2): 106.
-
Rejchrt, S., Cermak, P., Pavlatova, L., Mickova, E., and Bures, J. 2004. “Bacteriologic testing of endoscopes after high-level disinfection.” Journal of Hospital Infection 25(3): 76.
-
Richard, M. and Pineau, L. 2011. “Evaluation of a storage cabinet for heat-sensitive endoscopes in real use conditions.” American Journal of Infection Control 39(5): E18.
-
Riley, R., Beanland, C., and Bos, H. 2002. “Establishing the shelf life of flexible colonoscopes.” Gastroenterology Nursing 25(3): 114.
-
Schmelzer, M., Daniels, G., and Hough, H. 2015. “Safe storage time for reprocessed flexible endoscopes: a systematic review.” JBI Database System Rev Implement Rep 13(9): 187.
-
SGNA. 2018. Standards of infection prevention in preprocessing flexible gastrointestinal endoscopes. Society of Gastroenterology Nurses an d Associates.
-
Vergis, A., Thomason, D., Pieroni, P. and Dhalla, S. 2007. “Reprocessing flexible gastrointestinal endoscopes after a period of disuse: Is it necessary?” Endoscopy 39(8): 737.









