A User’s Guide to kNOw the Risks Associated with Reprocessing TEE Probes
It is not always obvious, but before embarking on almost any task, people undertake a risk assessment1. One weighs the possible risks against the potential rewards and bases their decision on what happens on that scale. Unfortunately, humans are not the best at risk assessment1; too many people use their intuition to determine the best path forward, as opposed to taking a scientific approach. The problem lies in the fact that one’s knowledge is limited and both sides of the scale are missing valuable data that could impact the outcome of the assessment.
When it comes to reprocessing transesophageal echocardiogram (TEE) ultrasound probes, one cannot afford to base decisions on gut feelings. The right way to determine what a facility’s standard procedure for reprocessing TEE probes is by gathering all the available data, analyzing each piece, and weighing the options. One must first know all the risks in order to ensure that there is no risk. This is not just important for a facility’s integrity and bottom line, but for the patients it serves.
It may seem simple, but reprocessing TEE ultrasound probes is quite an involved process, especially when trying to go about it the right way. There are ten individual elements that need to be examined carefully to determine and mitigate risk when reprocessing probes: transportation to and from the procedure room, bedside cleaning, cleaning, rinsing, high-level disinfection, electrical leakage testing, the drying method employed, storage of the probe, material compatibility, and staff.
Each one of these elements requires a significant amount of attention to every detail. If one step is skipped or inadequately performed, then the entire reprocessing procedure will be inadequate. This can result in serious and even life-threatening consequences. Failure to precisely carry out each step of the procedure can bring about risks to the TEE probe, the patient, and the facility.

When performing any step in the reprocessing procedure, it is possible to damage the probe handle, insertion tube, or distal tip. Damage to any of these components can result in different risks. For example, damage to the handle or the distal tip can result in the TEE probe malfunctioning, degraded images, or becoming nonfunctional.
If the TEE probe’s insertion tube is scraped or cut, it can harbor bacteria. Over time, that bacteria can form a biofilm, which is nearly impossible to remove from the insertion tube without causing further damage. Biofilms have been described as cities for microbes, enhancing and fostering growth and development. The Centers for Disease Control and Prevention (CDC) describe biofilms as, “Microbial communities that are tightly attached to surfaces and cannot be easily removed… Bacteria within biofilms are up to 1,000 times more resistant to antimicrobials than are the same bacteria in suspension.”2 Because it can withstand high-level disinfection, biofilm presents a very real danger to patients who are immunocompromised.
When an ultrasound probe is damaged or its safety is compromised in any way, it must be either repaired or replaced. Not doing so jeopardizes patient health and safety and can even put your own staff at risk. Unfortunately, repairs can cost thousands of dollars and replacements can be tens of thousands of dollars. Anything you can do to prevent damaging these delicate and costly probes is worthwhile.
Reprocessing failures can also result in risks to patients. If a TEE ultrasound probe is not fully and properly reprocessed, then it can harbor dangerous bacteria which can harm subsequent patients. The CDC estimates that about three percent of all hospital patients contract infections during their stay in the hospital; these are called healthcare associated infections (HAIs).3 In one of the most staggering statistics provided by the CDC from their most recent hospital survey, “About 72,000 hospital patients with HAIs died during their hospitalizations.”4 An improperly reprocessed TEE probe leads to significant risk for a facility. In this social media age, a facility’s reputation cannot afford mistakes. The Health Research Institute conducted a survey in which they found that about 25% of consumers have posted online about their healthcare experiences while 42% have used social media to access reviews of treatments, physicians, or facilities.5 These reviews posted by patients impact the reputation of a facility or doctor’s office significantly. As a result, each facility must constantly be providing the best service or risk ruining their name and standing in the community.

Failures in reprocessing can also lead to significant financial risk for a facility. Becker’s Hospital Review reports that, “The total direct, indirect and nonmedical social costs of HAIs are estimated at around $96 billion to $147 billion annually, including loss of work, legal costs and other patient factors.”6 Due to changes in the law, the Centers for Medicare and Medicaid Services (CMS) will no longer pay for certain preventable complications, which includes many HAIs.7 Now the entire financial burden will rest on the shoulders of each facility responsible for the transmission of an HAI.
Additionally, a facility may be held legally responsible for the transmission of an HAI. This could result in lawsuits and years of trouble ahead. In fact, Becker’s Hospital Review reports, “The typical hospital is the target of seven HAI-related lawsuits per year with an average settlement of $1.5 million.”8 The financial hazard associated with reprocessing TEE ultrasound probes is avoidable if steps are taken to mitigate the risks, but first, one needs to be aware of the risks. By carefully analyzing each of the ten elements previously mentioned, best practices can be incorporated into standard operating procedures, the best equipment for each job can be utilized, and risk can be significantly diminished. Ultimately, the goal of all this effort is improved outcomes for a facility’s reputation, finances and, most importantly, patients.

TPorter® TEE transport device and TransPorter™ mobile cart
Transportation
Best practices for TEE probe transportation from one area of a facility to another are not always utilized. Instead, less effective methods such as plastic bins, trash bags, and even pillow cases are sometimes used to transport these devices. Being expensive and highly sensitive, TEE probes should be handled in a much more delicate manner. Probes transported using these unsafe and ineffective methods are more likely to be damaged. In a fast-paced healthcare setting, staff could easily damage a probe by accidentally banging the distal tip against a doorframe or even dropping a bag that isn’t properly secured. Just a single scrape along the shaft or a hard bump against the distal tip and a probe could be damaged or, in the worst-case scenario, broken beyond repair.
Unfortunately, it is not always obvious when a probe has been damaged. This could result in a TEE procedure being carried out with a damaged probe. If a damaged probe is used on a patient, there are risks of incorrect readings, wasted time for the facility and patient, and even the possibility of electrocution to the patient which, according to the American Institute of Ultrasound in Medicine could have, “fatal results.”9
Additionally, these modes of transportation risk the contamination of a clean probe and make it more likely for a soiled probe to contaminate anything it comes into contact with. This is not safe for patients or staff. To mitigate the risk of damage or cross-contamination, facilities must utilize a device specifically designed for the safe transportation of clean and soiled TEE probes. The container should meet industry standard guidelines for containment and labeling to help prevent cross-contamination. The Joint Commission (TJC) requires that probes be transported in a container which is, “leak-proof, puncture-proof, and labeled as biohazardous.”10 The case should be tough enough to protect the ultrasound probe but light enough to comfortably carry from one room to the other.
One option is the TPorter TEE Probe Transportation and Procedure Case. The durable polycarbonate TPorter ensures that probes are not damaged during transportation to and from the procedure room. TPorter is a complete delivery system which has a compartment for everything needed for a TEE procedure and all the labels required to mark a clean or dirty probe. Facilities have reported a reduction of repair costs after introducing the TPorter. For example, according to Duke University Medical Center, mechanical failures have been reduced greater than 50% since utilizing the TPorter system. Implementing a TPorter into a facility’s standard TEE procedure can reduce risk to the probe, the facility, and patient health.

Enzymatic sponge applicator for pre-cleaning of soiled probe
Pre-Cleaning
Pre-cleaning—also called bedside or point of care cleaning—can be misinterpreted. Because it has so many names and sounds similar to the next step in reprocessing (i.e. cleaning), this step can be overlooked or performed improperly. This step is, however, of vital importance to the success of each of the following steps in the reprocessing procedure.
If pre-cleaning is not executed properly and at the right moment, significant risk is introduced to the procedure. Organic soils, bacteria, and pathogens from the patient would be left on the probe’s insertion tube and could begin to dry before the TEE probe is transported to the reprocessing area. By the time the probe is immersed in the cleaning solution, it could be too late. Depending on the amount of time between removal from the patient and cleaning, the bioburden left of the insertion tube could have hardened into a nearly impervious biofilm. Biofilm will protect the bacteria within from cleaning and even from high-level disinfection.
Removing biofilm is nearly impossible and could result in damage to the probe’s insertion tube. This risks the integrity of the probe and would result in a health risk to the next patient. Especially concerning is the fact that the next patient could be immunocompromised which could have severe implications should any bacteria remain on the probe. Skipping or inadequately executing this step could result in risks to the probe, the facility, and patients’ health.
The mechanical, or manual, wiping of the insertion tube with an enzymatic applicator begins the process of chemical breakdown of proteins, lipids, and other soils left on the TEE probe. It is critical to start this process within the first hour after completing the TEE procedure. This keeps any bioburden moist, preventing it from hardening into biofilm.
CS Medical’s TEEZyme Sponge, an enzymatic applicator, helps minimize the risk of the development of biofilm and helps ensure the success of the reprocessing procedure as a whole. TEEZyme Sponges are laden with a multi-tiered enzymatic detergent with a neutral pH, specifically formulated to remove gross contaminants and prepare the probe for the cleaning step. Once the probe has been wiped down with a TEEZyme Sponge, it can be covered with a PullUp Bio-Barrier Sleeve to keep it moist and safely transported to the reprocessing area in a TPorter. This simple solution protects expensive TEE probes, the facility’s reputation, and patients.
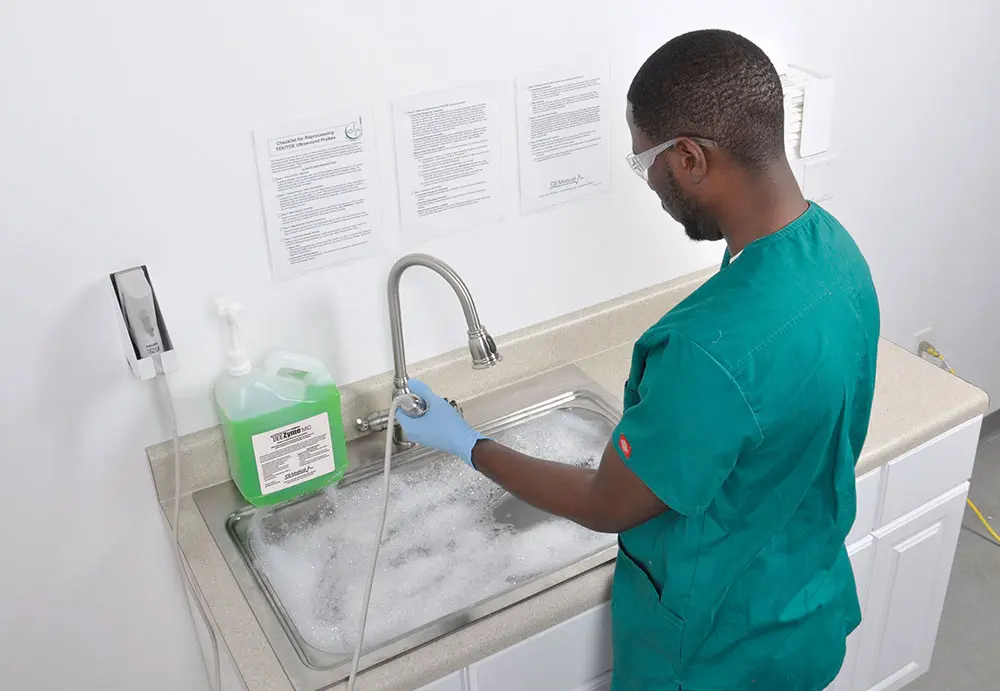
Cleaning
Another area of risk to carefully consider is found during the cleaning step. In order for a probe to be high-level disinfected, it must undergo cleaning. According to the Society of Diagnostic Medical Sonography (SDMS), “Effective disinfection or sterilization requires adequate cleaning.”11 Once again, what seems like a simple procedure is laden with risks if not performed with near-perfect precision.
To manually clean a probe, the pre-cleaned probe insertion tube must be submerged in a basin or sink filled with tempered water and the appropriate amount of enzymatic solution. The probe’s insertion tube must remain in this bath for the manufacturer’s directed time while concentration and temperature are kept steady. The TEE ultrasound probe must be closely watched throughout this time to ensure that neither the handle, the strain relief, nor the electrical connector slip into the bath or become soaked as this could irreparably damage the probe.
If correct contact time, temperature, and concentration are not met, the probe will not be properly cleaned. As one might imagine, these variables result in significant risk of failure for the entire reprocessing procedure which, in turn, puts patients and the facility at greater risk.
While encouraging those who reprocess probes to, “be careful,” might reduce risk slightly for this step, the only way to nearly eliminate this risk is by automating this step. Automation is widely recognized to mitigate risks associated with human error and yields better outcomes in almost all cases. The SDMS clearly states, “Automated processes are preferable due to the reduced risk of operator error.”12 CS Medical’s TEEClean® Automated TEE Probe Cleaner Disinfector completely automates the cleaning step, ensuring uniform results and better outcomes for patients and the facility.

Rinsing: Pre- and Post-High-Level Disinfection
Rinsing is critical to the success of the overall process and happens two times during the reprocessing procedure: once before and once after high-level disinfection. After a TEE probe has been cleaned, it must be rinsed to remove chemicals that could interfere with the high-level disinfection step. The TEE probe must then be rinsed again after high-level disinfection to remove any high-level disinfectant (HLD) from the probe. If any HLD is left on the probe’s insertion tube, this could pose a risk to the patient. HLDs are not safe to come in contact with and can cause negative reactions if exposure occurs.
Rinsing appears to be an easy process and, in some ways, it is: run the probe under water for a few minutes and you’re done. What gets overlooked is the quality of the water that is coming out of the tap, especially after high-level disinfection. A report from USA Today in 2016 found that almost 2,000 systems in the country had contaminants like, “excessive lead levels… bacteria, nitrates, arsenic and copper, all of which present potentially serious health risks.”13 Water supplies in healthcare settings are aging and could contain contaminants that should not be reintroduced to a newly reprocessed TEE ultrasound probe. Rinsing a disinfected probe with contaminated water can lead to bacterial growth. ASHRAE 188-2015 discusses contaminated water and states that a water management plan is essential, a minimum standard for any healthcare facility.14
Water quality matters when it comes to rinsing probes. Contaminants and bacteria in the water may attach to the probe and be introduced into the next patient, potentially causing an HAI. Rinsing a probe in contaminated water wastes all the time and effort that has been placed into reprocessing the probe. This costs time and resources which are already in short supply.
To mitigate the risks associated with rinsing probes in tap water, filtered water must be used. For rinsing disinfected probes, the CDC recommends, “If sterile water is not feasible, water that has been passed through filters with a minimum pore size of between 0.1 – 0.2 microns would be essential as a rinse fluid for this device.”15 CS Medical has engineered the TEEClean® Automated TEE Probe Cleaner Disinfector, to have an incorporated FDA-cleared 0.05 micron water filter for their rinse cycles. That is 40 times more filtration power than the CDC’s baseline recommendation. These water filters are also able to be used with the TD 100® Automated TEE Probe Disinfector and the TD 200® Automated TEE Probe Disinfector.
This is another case in which automation pays off. By automating the rinsing process, a facility can be certain that each probe receives the same level of care and attention each and every time. Each probe will be rinsed for the appropriate amount of time with the correct amount of filtered water. This helps protect patients and the facility from the risks associated with rinsing.

IAC (Intersocietal Accreditation Commission) recommends the structural and electrical integrity of the transducer be checked between each use, using an ultrasound transducer leakage tester.
Electrical Leakage Testing
Before TEE probes are used on a patient again, they must be tested for electrical leakage. Because TEE probes are such delicate instruments, they can be easily damaged. Unfortunately, damage is not always easy to spot. If probes develop a deep enough cut or crack in the insertion tube or distal tip, they could pose a significant risk to a patient or staff member.
When in use, TEE probes are connecting a 120 or 240 volt electrical source directly to the patient. Even the smallest crack in the insertion tube of an ultrasound probe can result in the electrocution of a patient which, according to the American Institute of Ultrasound in Medicine (AIUM), could have, “fatal results.”16 To make a horrible situation worse, the electrical current that would be sent through a patient could even make its way into the operator leading to an incredibly dangerous and potentially deadly situation.17
Reducing the risk of electrocution is a simple matter solved by electrical leakage testing between each and every use. Not only is electrical leakage testing simply a best practice, it is a standard required by various accrediting agencies. As of 2015, the Intersocietal Accreditation Commission (IAC) has required, “the structural and electrical integrity of the transducer must be checked between each use, using an ultrasound transducer leakage tester, ‘Passed’ or ‘Failed’ must be documented in the routine TEE probe cleaning/maintenance log along with action taken if ‘Failed.’”18
To meet this need, CS Medical offers an ultrasound leakage tester and respective adapters that are specific to each TEE probe. When used in conjunction with an automated reprocessor, electrical leakage testing can be completed within the reprocessor, resulting in more effective workflow and better time management. To reduce the risk of electrocution, facilities should always check for electrical leaks in between each use of a TEE probe. Testing keeps the facility compliant with best practices and protects patients’ well-being.

Automation of high-level disinfection with TD 100® Automated TEE Probe Disinfector.
High-Level Disinfection
The next step is probably the most complex and risk-filled step in the entire reprocessing procedure. The high-level disinfection step introduces risk to the probe, the patient, the facility, and even staff members.
Once a probe has been cleaned and rinsed, it must be high-level disinfected. The process of manual high-level disinfection is similar to the steps taken during manual cleaning, but with much stronger chemicals involved and longer contact times. Disinfection is accomplished by submerging the probe’s insertion tube in a bath of HLD solution for the manufacturer’s directed time while temperature is kept steady. The TEE probe must not be left alone throughout this time to ensure that the handle does not slip into the bath.
To ensure that the right concentration has been met or a reusable disinfectant has not degraded over time, the solution must be tested with a chemical indicator test strip, also called a minimum recommended concentration (MRC) test strip. In some cases, these strips can be hard to read, so they require careful analysis to confirm the MRC has been met. If the solution is too weak, the probe will not be high-level disinfected; if the solution is too strong, the probe’s insertion tube may be damaged by the harsh chemicals.
The solution must be raised to a temperature determined by the disinfectant’s manufacturer and kept at that temperature for the entirety of the high-level disinfection process. If the solution is not kept within the appropriate temperature range, the reprocessing procedure will be a failure.
When the solution is ready, the probe’s insertion tube can be immersed. The person reprocessing the probe must stay and monitor the probe to ensure the proper contact time and temperature is achieved and that the probe handle does not slip into the disinfectant. Suffice it to say that most staff members have more important work to do than sit around staring into a basin of disinfectant. Not only is that a waste of an employee’s valuable time, but it is not the best strategy for keeping staff engaged and attentive which leads to more frequent mistakes.
On top of all that, this step can actually pose a risk to the staff member monitoring the probe. Sustained exposure to the fumes produced by HLDs, like glutaraldehyde, can result in irritation to the eyes, nasal passages, or skin. According to an article in Infection Control Today, “In more serious cases, workers have attributed glutaraldehyde use to multiple chemical sensitivity/idiopathic environmental intolerance, resulting in litigation being brought against some healthcare facilities.”19 Worse, the CDC reports that using HLDs while pregnant may increase the risk for pre-term birth and even miscarriage.20 Spending time monitoring basins of disinfectant is not the best use of time and is also a serious risk for staff members charged with that duty. Many associations have standards on how to handle high level disinfectants, such as AAMI ST58, due to the hazards associated with using HLDs.21
There are many variables associated with high-level disinfecting a TEE probe, many of which are hard to consistently maintain and nearly impossible to perform perfectly. If all these variables are not faultlessly met for each and every probe, there are significant risks for the probe, staff members, patients, and the facility. As before, the best way to minimize the risk of high-level disinfection is automation. Automated reprocessors like the TEEClean® Automated TEE Probe Cleaner Disinfector, the TD 100® Automated TEE Probe Disinfector, and the TD 200® Automated TEE Probe Disinfector prevent skin and vapor exposure to harmful disinfectants and make reprocessing a low-risk endeavor for everyone involved.
Infection Prevention and Control (IPC) Standard IC.02.02.01 states, “The organization reduces the risk of infections associated with medical equipment, devices, and supplies.” As has been shown, automation is the best way to comply with that standard as it is the most effective way of reducing risk of all sorts. A study on the effectiveness of automation at the University Hospital Muenster in Germany found, “Automated disinfection had a statistically significantly higher success rate of 91.4% (106/116) compared with 78.8% (89/113) for manual disinfection (P = 0.009). The risk of contamination was increased by 2.9-fold when disinfection was performed manually.” This means that when minimizing risk is the priority, automation must be the method of choice when reprocessing TEE ultrasound probes.

QwikDry® used for removing a high-level disinfected TEE probe from the TD 100®, TD 200®, or TEEClean®
Drying: Pre- and Post-High-Level Disinfection
Drying is another step which must be performed twice: once before high-level disinfection and once after. After the probe has been cleaned and rinsed, it must be dried before high-level disinfection to ensure the HLD solution is not diluted at all. Then, once the TEE probe has been high-level disinfected and rinsed, it must be thoroughly dried again before it can be stored. If the probe is not dried, the wet surface allows airborne contaminants to stick to it and fosters the growth of spores and bacteria.
Drying is yet another step in TEE probe reprocessing that carries the potential for risk if not performed properly. One study reports that a full, “12.9% of transducers are contaminated with pathogenic material following disinfection.”22 That’s 12.9% of patients who are being exposed to potentially dangerous bacteria.
The prevalence of contamination of probes after disinfection is at least in part attributable to ineffective drying methods. Facilities often employ different methods for drying TEE probes after disinfection and rinsing; these methods include paper towels, reusable cloths, and gauze. There are risks associated with each of these methods.
One issue arises from the fact that most of these are likely to introduce bacteria onto the newly disinfected probe. Paper towels are often handled by various people before they arrive at their dispensers and are typically packaged in rolls or stacks. Reusable cloths, although relatively clean when they are first removed from their packaging, quickly become contaminated and harbor bacteria deep within, making them nearly impossible to decontaminate. With the exception of gauze, none of these come individually packaged, so they are susceptible to contamination from the environment they are kept in.
Another issue is friction. Paper towels can be coarse and abrasive. When a paper towel is wiped against a TEE probe’s lens, the coarseness of the paper towel can cause scratching, degrading image quality over time. Cloths themselves are typically non-abrasive, but it’s very easy for small particles and debris to be caught in the cloth’s fibers; this makes it risky to rub along the delicate probe and lens as any debris caught within could scratch the TEE probe. Gauze, while sterile, like cloths is not well-suited to dry probes because the fabric does not slide smoothly along the probe’s insertion tube. As a result, it might be tempting to cut corners and improperly dry the probe.
Lastly is the issue of what some of these drying methods leave behind. Paper towels leave behind tiny fibers along the insertion tube while gauze and other cloths leave lint. The fibers and lint left on the probe will eventually find their way into the next patient’s esophagus.
The best way to eliminate the risks associated with drying TEE probes is by drying them with a single-use, gamma irradiated product. This will minimize the reintroduction of contaminants to the TEE probe, ensuring that all the work done up to this point was not in vain. This would also apply to the previously mentioned IPC standard IC.02.02.01, where TJC charges facilities to, “…reduce the risk of infection associated with medical devices.”
QwikDry® Ultrasound Probe Drying Cloths are a great way to mitigate the risks in this step of the reprocessing procedure. QwikDry cloths are each individually packaged and gamma-irradiated. This means that the bacteria on the cloths has been eliminated, so they will not be a source of contamination to a recently disinfected TEE probe. Furthermore, these cloths are highly absorbent and have an ultra-smooth surface so they will easily glide over the probe’s insertion tube and lens without causing damage. Utilizing QwikDry cloths to dry probes reduces the risk of damage to probes and infection to patients.

CleanShield® TEE Probe Storage Cabinets are designed to safely and securely store disinfected TEE probes.
Storage
Proper probe storage has long been an issue of debate. Until somewhat recently, there were no widely accepted practices or standards for TEE probe storage. One introduces various risks if a probe is improperly stored. As a result, some obviously impermissible choices for probe storage include pillowcases, countertops, and hanging in the open-air. Some facilities store TEE probes in the manufacturer’s carrying case, however probe manufacturers explicitly state that their carrying cases are not meant for storage. The way TEE probes are stored is of vital importance for retaining their high-level disinfection status and, thus, to patient safety.
One common storage choice is a PVC pipe mounted on a wall. This option is far from low-risk as probes can be easily contaminated by being touched or via airborne contamination since the pipe has no closure at the top or bottom. Disinfection and drying must also be considered as part of risk management when using PVC pipes for storage. If the pipes are not disinfected between uses and thoroughly dried, one runs the risk of contaminants existing in the PVC pipes. Probes can also be at risk of damage if the pipe is mounted in a high-traffic area. Passersby can accidentally bump against the probe or pipe and cause damage to the handle, cable, or electrical connector. Additionally, because there is no way to lock or secure probes when using PVC pipes for storage, there is no way to regulate access to the probes so anyone can walk off with a probe without first checking it out. If any of these possibilities were to occur, it could cost a facility thousands of dollars in repairs, replacements, or compensations to a patient.
The SDMS provides some guidance on what probe storage should look like: “Proper storage reduces the risk of re-contamination of the transducer from environmental contaminants or accidental contamination during storage.”23 Both the CDC and TJC recommend that probes be stored hanging vertically with TJC specifying that they be in, “a clean, well-ventilated and dust-free area.”24 25
One form of probe storage meets the above requirements while providing solutions to most of the risks: a HEPA-filtered storage cabinet. Options like the CleanShield® TEE Probe Storage Cabinet are closed off from the environment and are constantly filtering the air within the cabinet, minimizing airborne contaminants. Contaminating a disinfected probe is unbelievably easy; just a tap or brush against another surface can soil a probe. According to one study, a TEE probe stored within a CleanShield should retain its high-level disinfected status for up to seven days.26
Cabinets also protect probes from mishaps. If, for example, someone were to accidentally trip and bump into the cabinet, all the probes within would be absolutely safe from damage or bacteria. A final point of consideration should be the overall construction of the cabinet and its ease of cleaning and maintenance. The cabinet’s material of construction in relation to the cleaning agents to be used should be considered in addition to the other standards as outlined by TJC and SDMS. Mitigating the risks of probe storage is as easy as storing probes inside a CleanShield Ultrasound Probe Storage Cabinet.

Transducer damage due to impact on the distal tip

Biofilm formation due to improper cleaning technique
Material Compatibility
Another major source of risk in the reprocessing procedure stems from material compatibility. If the chemical agents or automated reprocessor being used for reprocessing TEE probes are not compatible, there can be very serious consequences. This can happen as a result of seeking lower-cost options when it comes to cleaning solutions and HLDs.
In an effort to stretch funding, a facility may choose to purchase off-brand and alternate versions of cleaners and disinfectants, but the money saved may wind up actually costing more. If, for example, an HLD is used which has not been tested for material compatibility with a particular probe or with the reprocessor it will be used with, it may prove too weak or too strong to accomplish high-level disinfection. If the HLD does not have the correct MRC, it will result in a probe which has not actually been disinfected, leading to a health risk for patients and wasted time on the part of the facility. If the HLD is too strong, it can damage the surface of the probe’s insertion tube and permanently damage the probe which will require costly repairs. Worse, if the damage is minor and goes unnoticed, it could result in a compromised insertion tube that is much harder to reprocess and could become a health hazard.
The FDA provides TEE ultrasound probe manufacturers with an extensive guidance document outlining the requirements for material compatibility.27 For a manufacturer to attain FDA clearance, their probes must be rigorously tested for material compatibility and they must submit efficacy data, a record of how effective various HLDs and reprocessors are with their probe. It is critical for facilities to ensure that the TEE probe models they employ have been tested against the chemicals and reprocessors they will use to clean and high-level disinfect.
To mitigate the risks of material compatibility issues, facilities must only buy products which have been tested and cleared for material compatibility with their TEE probes. Companies like CS Medical are intentional about working alongside TEE probe manufacturers to make certain that their probes are compatible with their entire suite of products. This prevents damage to probes and safeguards patient health which reduces risk and saves facilities time and money.

Staff Members
The final area of risk found within the reprocessing procedure is with those who are tasked with reprocessing TEE probes. The risks range from improper training to simple human error.
According to the 2021 National Healthcare Retention and RN Staffing Report, the hospital staff turnover rate currently stands at 19.5%, the highest recorded in the industry for over a decade.28 As a result of the high turnover rate in the healthcare industry, since 2014, the average hospital has turned over about 90% of its workforce. This means that new staff members are constantly being brought on board and need to be trained. As has been demonstrated, the reprocessing procedure is extremely complex and must be carried out precisely. If staff members are not properly trained and following guidelines for annual training, they are more prone to make mistakes. To reduce repetitive tasks and mitigate the risks associated with them, automated sreprocessing should be implemented.
The task of setting up the proper reprocessing methods for TEE probes falls on the shoulders of infection preventionists. A challenge that infection preventionists must keep in mind is the nature of the process and that the same reprocessing method must be performed numerous times each and every day. This kind of repetitive exercise can cause fatigue and lead to staff members who are less attentive and careful about their work. Fatigue of this sort can have serious implications when dealing with the reprocessing of semi-critical devices. When the reprocessing procedure is not executed seamlessly, a patient’s well-being could be jeopardized.
The CDC states that healthcare facilities must keep staff members up to date and well-trained on the reprocessing of semi-critical devices.29 On-demand and in-person training offered by manufacturers is helpful in meeting this requirement and making sure all relevant staff is well-equipped to handle the reprocessing procedure. With the evolution of technology, real-time training events such as webinars and web-based training modules have enhanced infection preventionists’ skills and healthcare facilities with the ability to provide required, critical staff training with very little disruption to the work day.
Another way to mitigate the risks posed by human error is automation. Automating as much of the reprocessing procedure as possible can keep staff members from getting stuck performing monotonous, repetitious work and becoming fatigued. This can free up more of their time to focus on patients instead of probes. On top of that, automation streamlines the whole reprocessing procedure which simplifies the process of training new employees while ensuring consistent results. Automating as much of the reprocessing procedure as possible is the simplest way to mitigate risk.

Conclusion
Having reviewed the nearly countless risks associated with the reprocessing procedure for TEE ultrasound probes, one is in a much better position to make decisions and changes to minimize those risks and remove uncertainty in the reprocessing method. It is a heavy task to weigh these risks appropriately and to identify the correct mitigation strategy. Reducing the risks of TEE ultrasound probe reprocessing is a considerable responsibility, but with all the information, making the right decisions becomes clear. One first has to kNOw the risks so that there is NO risk.
Checklist for Reprocessing TEE Ultrasound Probes
This checklist is provided as an outline of steps required to successfully reprocess a TEE ultrasound probe. These steps are intended to follow guidelines and recommendations from manufacturers of the ultrasound probe, high-level disinfectants and recognized governmental and professional bodies. This checklist should be used as a reference for individual healthcare infection control professionals. ALWAYS DON PROPER PPE!
Step 1: Point of Care - Bedside
At patient bedside, immediately after removal from patient, apply an enzyme to the probe shaft with either a cloth or other applicator to remove organic and inorganic matter. By performing this step, it aids in preventing drying of the gross material on the probe shaft which can lead to biofilm formation.
Step 2: Transporting to Clean and Disinfect
Transport the TEE probe to the area designated for performance of the manufacturer’s directed enzymatic cleaning steps, i.e. basin with tempered enzymatic solution. Caution should be taken to minimize exposure to the potential biological hazard of the dirty TEE probe as well as to protect the probe from damage. Consult probe manufacturer’s IFU for handling and care.
Step 3: Manufacturer Cleaning
Insert probe shaft ONLY into enzymatic solution and follow manufacturer’s IFU for soak time. Typically, 2-3 minute submersion times are recommended. WARNING: TEE probes are not liquid sealed, DO NOT submerge handle or cabling.
Step 4: Manufacturer Cleaning Continued
Rinse enzymatic solution from TEE probe and dry with a lint- and contaminant-free cloth. Consult probe manufacturer’s IFU for type of material to ensure no damage to probe.
Step 5: Electrical Leakage Testing
Perform electrical leakage testing per probe manufacturer’s guidelines. Each TEE probe has different measurements, so consult your probe manufacturer’s IFU.
- Basin Test:
After electrical leakage test, ensure the probe shaft is dry prior to insertion into high-level disinfectant. ONLY insert the probe shaft into the basin for electrical leakage testing and high-level disinfection.
- Automated TEE Disinfector:
Follow the IFU from the disinfector manufacturer to complete the necessary steps for electrical leakage testing.
Step 6: High-level disinfection of TEE ultrasound probe
- Automated Single-Use Chemistry:
Once the TEE Probe has been placed into the automated disinfector, follow the screen prompts to perform high-level disinfection and rinse of the TEE ultrasound probe. The device will have the operator enter into the microprocessor the following data: operator name or designation, probe name or designation, and chemical lot number. With single-use chemistry, the expiration date on the bottle should be observed and if the expiry date has occurred, the chemical should not be used. NO MRC test strip is required to validate the efficacy of the disinfectant as long as it is used within the shelf life as cleared by the manufacturer with the FDA.
Step 7: Rinsing of HLD Probe
- Automated Single-Use Chemistry:
Automatic rinsing is incorporated into the design to ensure removal of disinfectant residue.
Step 8: Drying of probe after removal from high-level disinfectant
- Automated disinfector:
Once the final rinse is complete, remove the probe from the device. Apply a drying cloth, single-use, lint-and contaminant-free, to the shaft as you remove the probe from the machine. Care should be observed not to hit or bang the distal tip of the TEE probe as this portion is the most delicate.
Step 9: TEE probe storage
Place the high-level disinfected and dried TEE probe into a storage cabinet designed to hang the handle, electrical connector and shaft vertically and freely. Make sure that the handle, cable and electrical connector remain separate from the insertion shaft. All portions of the TEE, other than the insertion shaft, have only been surface disinfected while the insertion shaft has been high-level disinfected. Attention should be given not to bang or hit the distal tip of the probe as this portion is the most delicate. Per TJC requirements, the storage cabinet should allow the probes to hang vertically and have HEPA filtered air bathe them during storage.
Step 10: TEE probe transport
Remove the probe from the storage cabinet and transport the TEE probe to the procedure room in a manner that ensures the probe the following conditions:
- Secure as to not drop or bang the probe in transit
- Within an enclosure designed not to allow cross-contaminants to be reintroduced onto the probe
- Enclosure designed to transport the probe without damaging due to over coiling or components hitting each other during transit
References
https://www.cdc.gov/infectioncontrol/guidelines/disinfection/efficacy.html
https://www.beckershospitalreview.com/quality/hospital-acquired-infections-by-the-numbers.html
https://www.usatoday.com/story/news/nation/2016/03/22/lead-in-drinking-water-epa-report/82128166/
https://acertaralabs.com/wp-content/uploads/2018/02/ProbeElectricalLeakage_Guide_Rev3.pdf
https://www.intersocietal.org/echo/standards/IACAdultEchocardiographyStandards2021.pdf
https://www.csmedicalllc.com/bacterial-contamination-of-ultrasound-probes
https://www.cdc.gov/infectioncontrol/guidelines/disinfection/healthcare-equipment.html
https://www.nsinursingsolutions.com/Documents/Library/NSI_National_Health_Care_Retention_Report.pdf
https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html
About The Authors

Tami Gomez
Copywriter

Jessica Tharrington
Inside Technical Sales Specialist

Kendall Ashe
Vice President, General Manager

Tom Fischer, PhD.
Chief Science Officer




